Progress and Challenges in Objectively Measuring Bone-Strengthening Physical Activity
International Journal of Applied Sports Sciences, Vol.28, No.2, pp.59-67
ⓒ Korea Institute of Sport Science
초록
Osteoporosis is characterized by a loss of bone density and strength resulting in increased risk of fracture. One promising method for preventing fractures is participation in bone-strengthening physical activity. While the importance of mechanical loading for bone health is understood, assessment strategies are limited. Most researchers measure metabolic loads rather than mechanical loads, but not all activities that improve metabolic health increase bone strength. The osteogenic properties of physical activity (e.g., magnitude of the load, rate at which the load is applied, dynamic and odd nature of the load, duration of loading session, and breaks between sessions) have not traditionally been directly measured in health outcomes and surveillance research. The lack of research in this area has slowed our understanding of exactly what dose of bone-strengthening physical activity to recommend to the public as well as how to prescribe exercise to reduce the risk of fractures. To understand the influence of mechanical loading on bone adaptation, measurement methods must capture multiple physical activity dimensions (intensity, frequency, and time). Advancements in accelerometer technology now allow for the measurement of these dimensions. It is time that the lessons learned from using accelerometers in cardiometabolic health outcomes research be applied to musculoskeletal health.
Abstract
Osteoporosis is characterized by a loss of bone density and strength resulting in increased risk of fracture. One promising method for preventing fractures is participation in bone-strengthening physical activity. While the importance of mechanical loading for bone health is understood, assessment strategies are limited. Most researchers measure metabolic loads rather than mechanical loads, but not all activities that improve metabolic health increase bone strength. The osteogenic properties of physical activity (e.g., magnitude of the load, rate at which the load is applied, dynamic and odd nature of the load, duration of loading session, and breaks between sessions) have not traditionally been directly measured in health outcomes and surveillance research. The lack of research in this area has slowed our understanding of exactly what dose of bone-strengthening physical activity to recommend to the public as well as how to prescribe exercise to reduce the risk of fractures. To understand the influence of mechanical loading on bone adaptation, measurement methods must capture multiple physical activity dimensions (intensity, frequency, and time). Advancements in accelerometer technology now allow for the measurement of these dimensions. It is time that the lessons learned from using accelerometers in cardiometabolic health outcomes research be applied to musculoskeletal health.
Introduction
Osteoporosis is characterized by a loss of bone density and strength resulting in increased risk of fracture. It causes more than 8.9 million fractures annually worldwide in both men and women, resulting in an osteoporotic fracture every three seconds (Johnell & Kanis, 2006). It has been estimated that one in three women, and one in five men, over the age of 50 will experience osteoporotic- related fractures (Kanis et al., 2000; Melton, Atkinson, O’Connor, O’Fallon, & Riggs, 1998; Melton, Chrischilles, Cooper, Lane, & Riggs, 1992). These fractures often lead to chronic pain, loss of function, and loss of independence (Magaziner et al., 1997). Common sites of fracture are the forearm, hip, and spine. Hip fractures are reported to have up to a 24% mortality rate in the first year after fracture (Leibson, Tosteson, Gabriel, Ransom, & Melton, 2002). While men experience fewer hip fractures than women, their mortality rate is approximately double the mortality rate in the first six months after fracture as women (Kanis et al., 2003). The high incidence and mortality rates associated with hip fractures for both women and men make preventing fracture a prime target for health-related research. One promising method for preventing fractures is participation in bone-strengthening physical activity. The ability of physical activity to stimulate bone modeling and remodeling, also known as osteogenic potential, depends on the magnitude of the mechanical load, the rate at which the load is applied, the dynamic and odd nature of the load, the duration of the loading session, and the lengths of breaks between sessions (Baptista & Janz, 2012; Umemera, 2016).
While the importance of mechanical loading for bone health is understood, its assessment is limited. This is particularly true in health outcomes and surveillance research where most researchers measure metabolic loads rather than mechanical loads. In general, this approach indicates that as the metabolic intensity increases during physical activity so does bone strength (Physical Activity Guidelines Advisory Committee, 2008) (Table 1), but the relationship is not perfect and there are popular physical activity exceptions, e.g., swimming and bicycling. In addition, the other properties of physical activity associated with bone strength, e.g., odd loading, are seldom considered. Not directly measuring the specific osteogenic properties of physical activity in health outcomes and surveillance research has slowed our understanding of exactly what dose of bone-strengthening physical activity to recommend to the public as well as how to prescribe exercise to reduce the risk of fractures.
Table 1.
Physical activity intensity based on oxygen uptake and ground reaction forces.
| Oxygen Uptake | Ground Reaction Forces | Criteria | Examples | ||
|---|---|---|---|---|---|
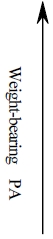 |
4-8 METs |
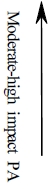 |
≥ 5 Í BW | Activities including jumping actions | Volleyball… Jumping (with or without counter movements, and drop jumps)… |
| 7-8 METs | 2-5 Í BW | Activities including sprinting and turning actions and moderate impact actions | Tennis, jogging, running (general), jumping (lateral jumping, jumping jacks)… Stepping… |
||
| 2-6 METs | 1-2 Í BW | Weight-bearing activities (repetitive) Low impact loading | Walking, dancing… | ||
| 4-8 METs | < 1 Í BW | Non impact activities | Cycling, aquatic activities for leisure… | ||
Legend to Table 1. This table shows that usually in weight-bearing physical activity, the increase in metabolic intensity (expressed by oxygen consumption) corresponds to an increase in mechanical load (expressed in ground reaction forces). Activities that include running and jumping are considered high impact-loading (> 3 x body weight). Activities that are intense in nature and include rapidly accelerating and decelerating movements, often in directions the body is not familiar with, are considered odd-impact.
In: Baptista, F. & Janz, K. F. (2012). Habitual physical activity and bone growth and development in children and adolescents: A public health perspective. In V. R. Preedy (Ed.), Handbook of Growth and Growth Monitoring in Health and Disease (pp. 2395 – 2411). New York, NY: Springer.
Currently, accelerometry is the most common type of objective physical activity measure in health outcomes (Kwon, Janz, Letuchy, Burns, & Levy, 2015; Shiroma et al, 2015; Vallance, Boyle, Courneya, & Lynch, 2015) and surveillance research (Manns, Ezeugwu, Armijo-Olivo, Vallance, & Healy, 2015). Accelerometer measurement is relatively inexpensive, places a minimal burden on participants, and provides physical activity dimension measures of duration, frequency, and intensity of movement. Previous researchers have recognized that these dimensions not only apply to cardiometabolic health, but also to musculoskeletal health (Table 2). This paper provides a brief overview of the properties of physical activity which influence bone adaptation and then explores the existing literature that has used accelerometers to better measure these properties. The purpose of this paper is to advance health outcomes and surveillance research addressing bone health by providing state-of-the-art information on how and what to measure to capture bone-strengthening physical activity.
Table 2.
Description of Accelerometer Features by Study.
| Author, Year | Brand | Model | Axes | Dynamic Range | Sampling Frequency | Wear Location | Type of Activities | Main Results |
|---|---|---|---|---|---|---|---|---|
| Janz et al., 2003 | CSA/ActiGraph | 7164 | Vertical | 0.5 – 2.0 g | 15 sec epochs | 1- Right hip | Walk, run, jump | Correlation coefficients between movement counts and GRF moderate to high for walking and running, not jumping |
| Garcia et al., 2004 | CSA/ActiGraph | 7164 | Vertical | 0.5 – 2.0 g | 10 sec epochs* 2 sec epochs** |
2- Waist | Walk, run, jump rope, drops | Counts from all accelerometers are associated with oxygen utilization; MML and CSA exhibited stronger relations than BioTrainer |
| AMI | Mini-Motionlogger | Triaxial | Minimum of 0.01 g | Not reported | 2- Waist | |||
| IM Systems | BioTrainer | 45° (vertical&horizontal) |
Minimum of 0.026 g | Not reported | 2- Waist | |||
| Ahola et al., 2010 | Developed by research group | Newtest | Vertical | Maximum of 11 g | Not reported | 1- Waist | Daily activity | Both exponential and logarithmic daily impact score can be used in acceleration-based measurements of daily exercise |
| Neugebauer et al., 2012 | IM Systems | BioTrainer | 45° (vertical&horizontal) |
Minimum of 0.026 g | 15 sec epochs | 1- Right hip | Walk, run | A mixed model regression equation can be used to estimate GRF from hip acceleration |
| Rowlands & Stiles, 2012 | ActivInsights, Inc. | GENEA | Triaxial | ± 8 g | 80 Hz | 1- Right hip 1- Right wrist |
Walk, run, jumps, drops | Counts and raw output correlate positively with GRF. Wrist-worn accelerometers show similar relationship with GRF as hip- worn monitors |
| Acti Graph |
GT1M | Vertical | ± 3 g | 1 sec epoch | 1- Right hip | |||
| ActiGraph | GT3X+ | Triaxial | ± 6 g | 100 Hz | 1- Right hip 1- Left wrist |
|||
| Stiles et al., 2013 | ActivInsights, Inc. | GENEActiv | Triaxial | ± 8 g | 100 Hz | 1- Right hip 1- Right wrist |
Walk, run, sweep, jumps, drops | Accelerometers worn at the hip or wrist can identify loading rates beneficial to bone in premenopausal women during daily activity |
| ActiGraph | GT3X+ | Triaxial | ± 6 g | 100 Hz | 1- Right hip 1- Right wrist |
|||
| Neugebauer et al., 2014 | ActiGraph | GT3X+ | Triaxial | ± 6 g | 100 Hz | 1- Right hip | Walk, run | Repeated measures generalized regression equations can estimate GRFs without a force plate |
| Pouliot-Laforte et al., 2014 | ActiGraph | GT3X+ | Triaxial | ± 6 g | 60 Hz | 1- Right hip | Hop, jump, heel-rise, chair-rise | Accelerometers are promising tool to assess GRF in everyday life |
| Meyer et al., 2015 | ActiGraph ActivInsights, Inc. |
GT3X+ GENEActiv |
Triaxial Triaxial |
± 6 g ± 8 g |
100 Hz 100 Hz |
1- Right hip 1- Right hip |
Walk, jog, run, drops, jump rope, break dance | Accelerometers that allow raw signal detection are reasonably accurate to measure impact loading of bone in children, but system -atically overestimate GRF |
| Deere et al., 2015 | GCDC | X16-1c | Vertical | Not reported | 50 Hz | 1- Right hip | Daily activity | Accelerometers can successfully distinguish physical activity impact levels in active and sedentary older adults |
Bone Adaptation to Physical Activity
Mechanical loading via physical activity has long been regarded as a means to influence bone mass and strength (Frost, 2003; Frost & Schonau, 2000). Joseph Wolff recognized the relationship between mechanical loading and bone reshaping in the late 1800s. His theory, Wolff’s law, stated, “Every change in the form and function of a bone or of their function alone is followed by certain definite changes in their internal architecture, and equally definite secondary alteration in their external conformation, in accordance with mathematical laws” (translation from German to English by Rasch and Burke (1978, p. 496)). Harold Frost later refined Wolff’s law with the Mechanostat theory (1987) where he proposed the existence of a homeostatic regulatory mechanism that controls building or resorption of bone in response to mechanical loading. He theorized that if the mechanical load remains below a certain threshold, bone is resorbed and excess mass is removed. However, if this threshold is exceeded and the bone is exposed to higher than normal mechanical load, bone building occurs to increase bone strength (Frost, 1987).
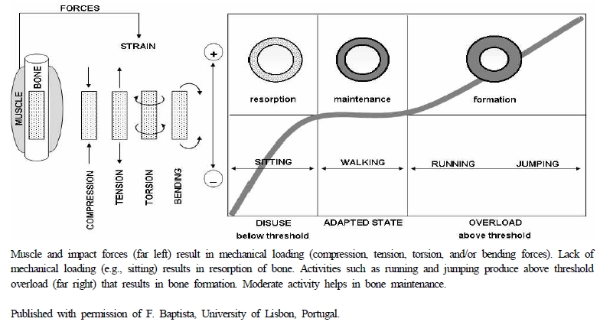
In 2004, the American College of Sports Medicine (ACSM) released a position stand titled, “Physical Activity and Bone Health.” Based on existing literature, they concluded that physical activity plays a vital role in bone health at any age. During childhood and young adulthood, physical activity plays a role in increasing bone mass until peak bone mass is obtained. This is supported by a systematic review of randomized and nonrandomized controlled trials of children and adolescents that indicated that exercise-induced gains in bone mineral density over six months at the femoral neck and lumbar spine ranged from 1% to 6% before puberty and from 0.3% to 2% during adolescence (Hind & Burrows, 2007; Weaver et al., 2016). Later in life, physical activity takes on the role of maintaining bone mass, attenuating bone loss with aging, and reducing falls and fractures (Kohrt, Bloomfield, Little, Nelson, & Yingling, 2004). This is supported by a systematic review and meta-analysis by Nikander and colleagues (2010) that found a general consensus that low-to moderate-impact weight-bearing exercise in combination with progressive resistance and/or agility training tends to be the most effective for improving hip and spine bone mineral density (or preventing bone loss) and functional ability in both older men and women. However, to influence bone health, the physical activity must comply with the principles of specificity and overload. Specificity refers to the fact that only skeletal sites that are exposed to a change in daily mechanical loading forces will undergo adaptation. Overload means that an adaptive response will occur only when the loading stimulus exceeds usual loading conditions (Kohrt et al., 2004). Both animal and human studies have shown that jumping provides an odd, non-repetitive overload that is effective at building bone strength. In rats, five jumps per day resulted in a significant increase in bone mass compared with no jumps per day (Umemera, Ishiko, Yamauchi, Kurono, Mashiko, 1997). In humans, Kato and colleagues (2006) instructed their exercise-training group to perform 10 maximal jumps per day three times per week for six months and saw significant increases in lumbar spine bone mineral density compared to baseline. No significant changes were seen in the control group. Additionally, dividing repetitions of mechanical loading into several sessions may have a greater effect on bones than completing all repetitions in one session. Robling and colleagues (2001) manipulated the recovery times among four daily, identical, 90-repetition mechanical loading sessions in rats and found that the bone formation rate in the 8 hour recovery group was 100% greater than that in the 0 or 0.5 hour recovery group. This supports the need for breaks between loading sessions. We direct readers to several reviews (Nikander et al., 2010; Kelley, Kelley, & Kohrt, 2013; Zhao, Zhao, & Zhang, 2014) as well as Figure 1 for more information on bone adaptation to physical activity.
Figure 1.
Muscle and impact forces (far left) result in mechanical loading (compression, tension, torsion, and/or bending forces). Lack of mechanical loading (e.g., sitting) results in resorption of bone. Activities such as running and jumping produce above threshold overload (far right) that results in bone formation. Moderate activity helps in bone maintenance.
Use of Accelerometry to Measure Mechanical Load
To understand the influence of mechanical loading on bone adaptation, measurement methods must capture multiple physical activity dimensions (intensity, frequency, and time). To measure the intensity of a given activity, the internal force applied to the bone must be quantified. Force is an entity, that when applied to a mass, causes it to accelerate. Newton’s second law of motion states that the magnitude of a force is equal to its mass times acceleration (F = ma). In animal research, bone strain magnitude is shown to be linearly proportional to the magnitude of the external load applied (Hsieh, Wang, & Turner, 1999). Therefore, the use of an external measure of load, such as ground reaction force (GRF), as an estimate of internal force is warranted. The current accepted measurement system for quantifying mechanical loading GRFs is the use of force plates in a laboratory setting (Winter, 1990). However, advancements in accelerometer technology allow for measurement of (raw) acceleration gravitational units (g), which can also provide a measure of mechanical intensity. Frequency and time of a given activity are traditionally measured via observation, but can also be provided by the time-stamp feature of accelerometers. Additionally, accelerometers, when worn at the waist, are sensitive to the forces experienced at the clinically relevant hip skeletal site. Previous studies (Table 2) have investigated the use of accelerometers to measure mechanical load with favorable results. Correlation coefficients between vertical GRF and accelerometer output have been shown to be moderate to high (r = 0.33 to 0.99). However, the earliest studies were limited by the technology available at the time. The following section discusses the advancements in accelerometer technology that have occurred over the past decade.
Advancements in Accelerometer Technology
As previously mentioned, the osteogenic potential of a physical activity depends on the magnitude of the load, the rate at which the load is applied, the dynamic and odd nature of the load, the duration of the loading session, and breaks in loading (Baptista & Janz, 2012; Umemera, 2016). Advancements in accelerometer technology allow for these characteristics to be measured. Accelerometers have evolved from only measuring one axis of movement to having the ability to measure up to three different axes at the same time. This allows them to measure odd loading that is characteristic of bone-strengthening physical activity. The range of accelerations that they could measure tripled over the course of these studies, allowing for higher intensity (magnitude) activities to be measured. The sampling frequency increased as well over this time, changing from epoch-level data (i.e., one measurement every one second or greater) to the ability to take measurements 100 times per every one second. This makes accelerometers more fit to measure mechanical loading, as these activities can occur very rapidly in short bursts. They do not need to last over a period of minutes to be beneficial to health, as activities that increase cardiovascular health do.
Accelerometer Variables
With the advancing technology in accelerometers over the years, the number and complexity of possible variables have also increased. In the early 2000s, when Janz and colleagues (2003) and Garcia and colleagues (2004) carried out their studies, the only variable available was activity counts (or movement counts) that were created by the accelerometer companies. The exact methods used to derive those counts were proprietary, and were not available to individual researchers. These counts also varied from company to company, so direct comparison between accelerometers could not be done with confidence. More importantly, these counts have traditionally been used to estimate energy expenditure (Crouter, Kuffel, Haas, Frongillo, & Bassett, 2010; Crouter, Flynn, & Bassett, 2015; Freedson, Melanson, & Sirard, 1998; Freedson, Pober, & Janz, 2005; Hildebrand, Van Hees, Hansen, & Ekelund, 2014), and were not derived for the purpose of measuring mechanical load.
A few years later, technology had advanced enough to allow researchers access to the raw g values. Ahola and colleagues (2010), Neugebauer and colleagues (2012), Pouliot-Laforte and colleagues (2014), Neugebauer and colleagues (2014), Meyer and colleagues (2015), and Deere and colleagues (2015) all took advantage of this advancement and utilized the raw, vertical acceleration g values. However, this also leads to issues of participants sometimes wearing accelerometers upside down, leading to inverted data. This problem can be remedied as long as researchers recognize the negative values in the data.
Once triaxial accelerometers were developed, researchers could choose if they wanted to use one, two, or three axes. Rowlands and Stiles (2012) and Stiles and colleagues (2013) utilized the vertical-axis only as well as all three axes in combination. The three axes are combined using a vector magnitude equation (sqrt (x² + y² + z²)) and are referred to as the resultant acceleration. This strategy has the advantage of utilizing all of the data from all three axes as well as not needing to worry if a participant wears a monitor upside down. It is also preferable to proprietary activity counts and using the vertical axis only because it utilizes as much of the raw data that is collected as possible.
Study Protocols
While the goal of all of these studies was to determine if accelerometers could be used to measure mechanical load, each used a different protocol. The majority utilized a criterion measure and had the participants complete various activities on the force plate while wearing accelerometers. Some studies used just walking and running at various speeds for the activities (Neugebauer, Hawkins, & Beckett, 2012; Neugebauer, Collins, & Hawkins, 2014). Some also included various types of jumps and drops in addition to walking and running (Janz, Rao, Baumann, & Schultz, 2003; Garcia, Langenthal, Angulo-Barroso, & Gross, 2004; Rowlands and Stiles, 2012; Stiles, Griew, & Rowlands, 2013). Others included additional activities such as the heel-rise and chair-rise tests (Pouliot-Laforte, Veilleux, Rauch, & Lemay, 2014) or break dancing moves (Meyer et al., 2015). The two studies that did not utilize a force plate had participants wear the accelerometers for a specified amount of time and then developed a daily impact score (Ahola, Korpelainen, Vaionionpaa, & Jamsa, 2010) or identified thresholds for defining high impact physical activity for future analyses (Deere et al., 2015). Each of these studies provided support for the use of accelerometers to measure mechanical load, but acknowledge that additional work is needed.
Future Directions
To accurately measure mechanical load via accelerometry, newer accelerometers that are triaxial, have a wide dynamic range, and a high sampling frequency are necessary. This will allow them to capture the high intensity, odd loading, and short nature of bone-strengthening physical activities. Criterion-based algorithms should be developed that are tested on a wide-range and intensity of bone-strengthening activities. Once these algorithms are developed in a laboratory settings, they should be validated in free-living situations where they will ultimately need to be used. Given the burden of fractures in older adults and the clear evidence that physical activity can improve bone strength, the time has come to understand its optimal dose for public health guidelines and exercise prescription. This understanding requires new research strategies using objective monitoring of physical activity. It is time that the lessons learned from using accelerometers in cardiometabolic health outcomes research be applied to musculoskeletal health.
References
Handbook of Growth and Growth Monitoring in Health and Disease (, et al., , ). (2012). New York, NYSpringer. Baptista, F. & Janz, K. F. (2012). Habitual physical activity and bone growth and development in children and adolescents: A public health perspective. In V. R. Preedy (Ed.), Handbook of Growth and Growth Monitoring in Health and Disease (pp. 2395-2411). New York, NY: Springer., Habitual physical activity and bone growth and development in children and adolescents: A public health perspective, In, pp. 2395-2411.