The relationships between resistance training and bone mineral density of the elderly: A meta-analytic approach
Article information
Abstract
The purpose of this study was to investigate the relationship between resistance training and bone mineral density of the elderly by using meta-analytic approach. A comprehensive literature review was conducted using such databases as EBSCOhost, Embase, and PubMed. A total of 12 articles were finally selected using the PRISMA procedure and analyzed using Comprehensive Meta-Analysis (CMA) 2.0. The results were as follows. Resistance training had a significant positive relationship on bone mineral density in lumbar, total hip and femoral neck. However, resistance training did not have a significant relationship on bone mineral density in trochanter.
Introduction
With the economic growth worldwide, the development of medical technology has extended human life expectancy. According to an announcement by OECD (2015), the average of expectancy life are approximately 80.5 and it has increased 10 years higher since 1970. Commonly, humans lose their physical abilities as they get older because of age-related diseases. Among diverse age-related diseases, osteoporosis is a typical disease with high incidence rates. Actually, patients with osteoporosis are estimated that there are more than 200 million people around the world (Aaseth, Boivin, & Andersen, 2012).
Generally, humans’ muscle strength and bone mineral density gets lower as age increases, so it is easier for older people to suffer from fractures caused by falling accidents (Marques et al., 2011). The number of fractures of the elderly is increasing in reality and especially the fracture in the spine and hips cause complications such as chronic disease, disability, a fall in quality of life and premature death (Dhanwal et al. 2011; Johnell & Kanis 2005). Moreover, Nair (2005) has asserted that decrease of muscles from the elderly shows up by decline in physical activity and because of lower stamina bone mineral density is weakened so in the end daily life becomes difficult and it also causes chronic degenerative diseases.
Osteoporosis depends on genetic factors, age, gender, dementia, very low number of BMI, lack of vitamin and calcium, low body activity, absence of estrogen, smoking and excessive alcohol consumption, drug abuse, etc. (Warburton, Nicol, & Bredin, 2006). Meanwhile, decrease of bone mineral density can lead to osteoporosis so preventive maintenance such as healthy eating and physical activity will be important. In fact, since 80s in academia, research has been made about effective exercise which increases bone mineral density and out of these weight bearing exercise and progressive resistive exercise are known to be effective (Marcus, et al, 1992; Sinaki, 1989). According to ACSM(American College of Sports Medicine) an organization known global for its kinetic medicine and sports science, adult over 50 year old are recommended to set up 8-10 set of exercises and to carry out resistance exercises with a frequency of 10-15 times per set.
Based on the prior studies, body weight exercise or physical activity increase the bone mineral density and the intensity, frequency, and duration of exercise is important to reduce bone mass reduction (Edward, Mark & Carl, 2000). Also, Suominen (2006) argued that because resistance exercise causes direct load to the bone and muscle action affects the bone increase or strengthen of the muscle means growth of the bone density.
Through these preceding studies, osteoporosis shows that a pathology mechanism exists and resistance exercise and physical activity are positive for the improvement of the bone density and can infer the importance of measurement of bone density. In addition, Kim & Kim (2008) disclosed to avoid osteoporosis drug treatment is available but it can only suppress bone loss not the increase of bone density and mentioned the significant of exercise to prevent. In other words, osteoporosis can increase muscle strength and bone density by non-drug treatment which is exercising and it can prevent the risk of fractures.
In this study, search for the research has been done on the effects of resistance exercise on the bone density of elderly populations, targeting old people who does not treat drugs. Also, considering the features of adaptation and change, more than 12 weeks of resistance exercise will be carried out and comparison of before and after of the exercise will be deducted finally according to the standard of PRISMA(Preferred Reporting Items for Systematic reviews and Meta-Analyses). PRISMA as an evolution of the original QUOROM guideline for systematic reviews and meta-analyses of evaluations of health care interventions. Meta-analysis is a study method that integrates and draws conclusion about a advanced research that covers particular subject and can be interpreted as an integrated study method.(Glass, 1977).
Actually, results of prior studies that has same topic they report different results and the effects vary widely. For example, research of Whiteford et al. (2010) if old people did resistance exercise for 6 months, bone density of all total hip has increased more than before the exercise but the group that did not exercise also rose to a certain extent. On the other hand, study by Marques et al. (2011) shows bone density increase in 8 months of resistance exercise however the figure of non-exercised group has decreased. Research by Kemmler and von Stengel (2013) measured elders bone density of total hip while 18 months of resistance exercise and it shows a high motion group had increase in bone density level before exercise and low motion group had decreased. Also, the number of figures dropped most from the control group.
Considering overall studies related to bone mineral density, even if the similar age group had the same movement the amount of effect differs. So, meta-analysis is necessary to integrate and analyze different results. The purpose of this study was to search for study of bone density and to integrate the effects of resistance exercise on the elderly’s' bone density by the meta-analysis. Especially, old people tend to get injury of spine, femur, and hipbone due to osteoporosis fractures and analyzed the lumbar, total hip, femoral neck, and trochanter which are primarily measured in the measurement of bone mineral density. Therefore, It was to summarize, as a starting point for developing future studies that higher quality of healthy life in elderly.
Method
Data collection
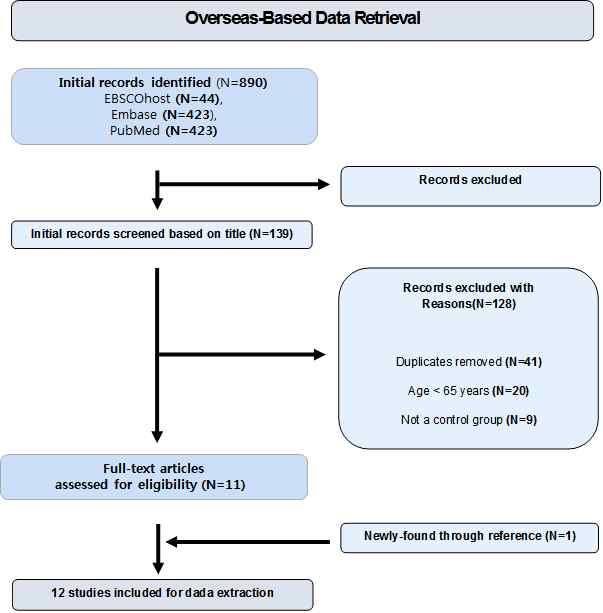
For the collection and analysis of the literature to be included in the meta-analysis, in this study out of several meta-analysis reporting basis, PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) reported by Moher, Liberati, Tetzlaff, & Altman (2009) has been followed. In this study, we include research which published in only English. Using ‘bone density’, ‘resistance exercise’, ‘weight training’, ‘geriatric’, ‘elderly’, ‘aged’, ‘older adults’, ‘bone mineral density’ as search words and search engines by EBSCOhost, Embase, PubMed the first 890 research was discovered. Primary removal of the studies which do not contain at least two of the previously mentioned search terms in the research title and 139 studies were selected. Next, analyzed the abstract of the previously selected researches 128 studies were excluded from analysis target due to suitability of the criteria set out in this study. Based on the references included in the final 11 studies which remained from the selection, one more study has been added so 12 studies(1995~2013) were selected for the meta-analysis at last. In the process of selecting and excluding research, based on the abstract between researchers and collaborators the decision was made after enough discussion and agreement, and the process of coding the final selected studies were done by three researchers individually and sections that do not match underwent confirm and modifications. <Figure 1> shows the summarized screening process of selected studies as a diagramming.
Selection and criteria
Out of previously described meta-analysis target study sorting process details related to conformity criteria are followings. First, age over 65 elderly should be the target, second, study should have controlled group set up, third, an elderly man who didn't receive hormone therapy or medication, fourth, number of samples should be over 10, fifth, an elderly man who doesn't have disease or disability, and last old people included in the treatment group should at least participated in resistance exercise for 12 weeks.
Measuring bone density
12 studies derived from this meta-analysis contained 13 bone density measuring parts. But each study had different measuring part so lumbar, total hip, femoral neck, and trochanter the repeated parts were established. Bone density was measured by Dual-energy X-ray absorptiometry (DXA, DPX) through X-ray and the unit of measure is g/cm2.
Statistical Procedure
In this study analytical program CMA (Comprehensive Meta-Analysis) which is used for meta-analysis was used. Described in the last 12 selected studies using treatment groups' average and standard deviation of bone density before and after, and controlled groups' average, standard deviation and number of samples bone density effect size was draw to a conclusion. In this study, to deduct effect size standardized mean difference was used. According to criteria by Cohen (2013) effect size is interpreted as about .8 is big and .5 is middle and .2 is small.
In order to statistically aggregate the effect size derived from individual studies homogeneity appraisal precedes precedence. Homogeneity appraisal is to see if effect size can be derived as the same population from each study by statistically appraisal in this study value of Q and I² carried out. As Lipsey & Wilson (2001) and Park, Shin, and Won (2015)s' study reports this research also considered the Q values' statistical significance and I² so if the homogeneity if rejected random effect model was used, and if it's not rejected fixed effect model was used. Also, we indicated that criteria of all statistical test was p<0.05
Results
Characteristics of individual studies
The basic characteristics of researches involved in this meta-analysis can be seen in <Table 1>. 12 studies were published throughout 1995 to 2013, the elderly who involved in treatment group participated at least 12 weeks of resistance exercise steadily and generally percentage of woman 60%, man 40%. Each individual studies depending on the purpose and characteristic of research each part of bone density was measured and out of those changes were reported in lumbar, total hip, femoral neck, and trochanter.
Publication bias
Before putting up individual effects together, this research has conducted a test that high effect size studies are highly likely to be published compared to low effect size studies which is publication bias. Publication bias check can be confirmed by funnel plot as reported in Bax, et al. (2009). Displaying funnel plot in diagram form for each lumbar, total hip, femoral neck, and trochanter, it is judged that the symmetry is approximately symmetry so it is seen publication bias isn't much involved likely as shown in Figure 2.
Meta-analysis
The results of total 4 parts of each bone density are the followings. First, out of 12 last selected studies lumbar bone density was included in 6 of the studies and experiment groups were 11. 360 elderly groups were involved in the exercise 341 people controlled the exercise. Lumbar bone density's effect size is .437 which is the highest out of 4 parts and confidence interval is .036~.838. It appeared statistically significant (Englund et al. 2005; Kemmler & von Stengel (2013); Marques et al., 2011; Pruitt et al., 1995; Taaffe et al., 1999; Whiteford et al., 2010). Second, total hip bone density was selected in 7 studies and experiment groups were 13. Elders who measured total hip part were 427 and 411 people controlled the exercise. Total hip bone density's effect size is .284 which is second largest. Confidence interval is .082~.486. So it means statistically significant(Allison et al. 2013; Kemmler & von Stengel, 2013; Marques et al., 2011; Pruitt et al., 1995; Taaffe et al., 1999; Whiteford et al., 2010; Woo et al. 2007). Third, femoral neck bone density was sorted in 9 studies and experiment groups were 12. Old people who measured femoral neck part were 360 and exercise was controlled by 359. The effect size is .239 and third highest. Confidence interval is .091~.387 and it appears statistically significant (Allison et al., 2013; Englund et al., 2005; Park et al., 2008; Pruitt et al., 1995; Rhodes et al., 2000; Vincent et al., 2002; Whiteford et al., 2010). Lastly, trochanter bone density was selected in 7 groups and experiment groups were 8. 300 old man measured trochanter part and exercise was controlled by 291 people. Effect size was .139 which is the lowest (Allison et al., 2013; Englund et al., 2005; Park et al., 2008; Rhodes et al., 1995; Whiteford et al., 2010). Confidence interval is -.023~.301 which isn't statistically significant.
Discussion
For decades studies have been consistently progressed to verify the effectiveness of the exercise to prevent and cure aging. Most of the studies done before claimed exercise and bone density had positive relationship and especially there is a result showing that resistance exercise helps to prevent fracture and makes bone density higher for elders (Hurley & Roth, 2000; Korpelainen et al., 2006). The purpose of this study is to search for researches about bone density which were targeted for the aged to see the effect of resistance exercise to bone density as meta-analysis. So, studies selected for this research applied various exercise ways for old people and its effect size had some differences or different results. Specific discussions on the results of the analysis are the followings.
First, resistance exercise had positive effect for elders lumbar bone density to go up. It is analyzed out of 4 parts it had the largest effect size which is .437 and with 11 experiment group. Other previous studies also mentioned postmenopausal women and above exercising program helps the bone loss from lumbar or recover about 1% for a year (Blanchet, et al., 2002). Also, Bolam, Skinner, Jenkins, Galvao & Taaffe (2016)s' study shows elders having resistance exercise for 9 months lumbar bone density has increase significantly. Lumbar is a bone that forms waist part out of the spine and it protects the spinal nerves and support human body so resistance exercise strengthening lumbar can be important for elders. Bone density going weaker and as aging goes on injuries like fractures of lumbar vertebrae is known as vulnerable diseases for old man and it happens because vertebral bone gets lower (Johnell & Kanis, 2006). Such injuries may depend upon the limit which makes the waist to stoop forward more and may complain of ache so it goes under medication therapy, wearing autopsies, and physiotherapy for the treatment. Bone density loss for aging will lead to bone fracture and a lot of synchronism. This research can be seen that the effect size of resistance exercise was the biggest for bone density moving the body is important for preventive action.
Second, resistance exercise had positive effect for total hip bone density to go higher. For results of 13 test groups effect size was .284 which was second highest out of measured parts. Kerr, Ackland, Maslen, Morton, & Prince (2001)'s study also shows when postmenopausal women and above did resistance exercise total hip bone density had a significant influence. On the other hand, Nicholson, McKen, Slater, Kerr & Burkett (2015)s' study showed when old people average age of 66 had resistance exercise for 6 months with low weight and high repetition lumbar and femoral neck bone density has increased but total hip bone density went down a bit. Of course the group that controlled exercise went down more than the group that did exercise. It should be considered that it was a low intensity motion which used low weight. Commonly, total hip fracture is around hip joint and it usually happens to people age over 70 who has serious osteoporosis and usually at winter. To avoid it physical condition to cope with the fall should be improved and especially strengthen of quadriceps muscle of thigh, biceps muscle of thigh, sense of balance and walking exercise. This is also good to be done by resistance exercise and especially for aged bearing wright exercise like walking up the stairs lightly would be good.
Third, resistance exercise for elders had positive effect in femoral neck bone density to go up. It was analyzed by 12 test group the effect size was .239 which was third highest. Previous study by Jessup, Horne, Vishen, & Wheeler(2003) when old woman did weight bearing exercises like walking up the stairs, walking, and balancing exercise for 32 weeks, femoral neck bone density had a significant influence. Gianoudis et al. (2014) also made old woman to do resistance exercise for 12 months the bone density of femoral neck had increased significantly.
Femoral neck fracture is one of total hip fracture that usually occurs to old people along with lumbar fracture. Femoral neck fracture is known different from other parts that femoral head avascular necrosis or femoral neck disharmony to be highly probable (Singh, Nagrath, & Maini, 1970). It is better rather than telling the elderly to sit quicker encouraging and improving them to do appropriate resistance exercise and balancing exercise to prevent from injury.
Lastly the effect of resistance exercise for trochanter bone density had very low effect size. In this study there were 8 test groups about trochanter. There were different results that had the bone density to go up or down and out of 4 parts the effect size was the lowest with .139 and it wasn't statistically significant. In previous studies Protiva(1997) had woman age over 75 to wear weighted vest and to do weight bearing exercise but it showed up that the effect wasn't significant to trochanter bone density.
Even though legs exercise was performed, Trochanter effect is low than other’s result. This result was maybe associated with training program such as group type, frequency and methods etc. The Resistance training is need to accompany with balance training for enhancing BMD, preventing risk of damage. Moreover plyometric, dynamic stability, proprioceptive exercises were recommended for elderly. It is best stragey for preactivation and cocontraction and they help muscle contractions through blood flow(Haff & Triplett, 2015). In this meta-analysis it doesn't mean that resistance exercise has no effect to trochanter bone density just because of low effect size. Other previous studies Kerr et al. (2001) had a result that 1 year of resistance exercise helped the elderly’s trochanter bone density to go up. In research of Korpelainen et al. (2006) 30 months of resistance exercise by postmenopausal elderly women showed protective and maintenance than exercise controlled group in trochanter bone loss. Compared to femoral neck fracture intertrochanteric fracture has a smooth flow of blood and a lot of spongy substance of bone which helps synostosis. However rather than treatment because of injury exercising appropriately and maintaining is necessary for prevention. According to Blain et al. (2004) keeping enough weight and having body fat can have a protective effect to prevent the bone loss. Also study by Pluijim et al. (2001) said suitable amount of fat for old women can have an effect to bone density. Assumed by this exercise controlled group in the study can't be controlled with the walking exercise and keeping the weight, body fat or eating habit might have some influence to the bone density of trochanter and other parts. So, it is thought that in the exercise controlled group the reason of elders bone density remaining the same or increasing slightly might be walking exercise, enough weight and the right eating habit might be continued or fixed.
Also general factors related to bone density are known as age, gender, family history, body activity, smoking, alcohol consumption, hormonal condition and weight etc. (Kanders & Dempster, 1988; Nguyen, Sambrook, & Eisman, 1998). Out of these weight is known representatively as being able to changed possibly and many of the previous studies showed high weight had high bone density, also when increasing the weight percentage of total hip fracture to occur went down (Langlois, Harris, Looker, Madans, 1996). But by changing weight cannot just increase the bone density. In the study by Yi, et al. (2001) it is said lean body and body fat which both constitute the weight may differ and has no idea which one has more influence to bone density. Overall many researchers proved that by body activity bone density can increase. In other words, when becoming old body activity may go down so speed of reconstruction of bones go slow which leads to low bone density. Partially, Aerobic exercises include stepping, graded walking, skipping, dancing, jogging etc (Marques, Wanderley & machado, 2011). By contrast, Resistance exercise were using weight machine, dumbbell, small tool weight load and etc. in this study. It can be integrated in to the aerobic + strength exercise.
In addition, in this study effect size appeared in order of lumbar, total hip, femoral neck, and trochanter. Because resistance exercise has work out movements that use relatively large area and waist and hip joint gets big stimulate the effect size were different. The Correlation between exercise and bone health has been extensively debated (Kohrt et al. 2004), By meta-analysis this study also analyzed the influence of resistance exercise to elders bone density. The perfect control of exercising group and exercising controlled group wouldn't have been done. However generally considered resistance exercise cause positive effect on senior and elder’s osteogenesis and increase of bone density.
Conclusion
Meta-analysis in this study integrated and analyzed the influence of resistance exercise to elders bone density. Resistance exercise had different effect size for each parts and it was bigger in lumbar, total hip, femoral neck, and trochanter order. In other words, resistance exercise had positive influence to elders bone density. Because it is judged that it has positive effect on bone density exercising appropriately and having right eating habits are necessary to make maximum osteogenesis from being young to maintain and strengthen it.
Although this study has some limitations, these will be the guidelines for the follow-up study. First, the results of this study cannot be generalized because we used researches which were published in English. If we have more researches about the elderly in diverse countries, it will produce more meaningful results. Second, meta-analysis in this study was selected by the purpose and PRISMA criteria. In this process the year of publication of individual studies has 20 years gap. Follow-up study should set up the publishing year as moderator and analyze. Third, This review is limited by the lack of relative design such as duration and a variety of exercise methods to old adults. Therefore, no reliable conclusions as to the effect of exercise for different duration and methods. Fourth, The majority articles have been studied in women as a result of preventing osteoporosis in this population. This is cause by menopause. Therefore, These studies in men are fewer and more research is required to know real effects of resistance training in the sex . lastly, It will be interesting for follow-up study to investigate the relationship between types of resistance exercise and the bone density.
References
Aaseth, J., Boivin, G., & Andersen, O. (2012). Osteoporosis and trace elements–an overview. Journal of Trace Elements in Medicine and Biology, 26(2), 149-152.
10.1016/j.jtemb.2012.03.017.ACSM (2000). American College of Sports Medicine. ACSM's Guidelines for Exercise Testing and Prescription (sixth edition). Lippincott William & Wilkins.
Allison, S. J., Folland, J. P., Rennie, W. J., Summers, G. D., & Brooke-Wavell, K. (2012). High impact exercise increased femoral neck bone mineral density in older men: a randomised unilateral intervention. Bone, 53(2), 321-328.
10.1016/j.bone.2012.12.045.Bax, L., Ikeda, N., Fukui, N., Yaju, Y., Tsuruta, H., & Moons, K. G. (2009). More than numbers: the power of graphs in meta-analysis. American Journal of Epidemiology, 169(2), 249-255.
10.1093/aje/kwn340.Blain, H., Carriere, I., Favier, F., Jeandel, C., Papoz L., & EPIDOS study Group. (2004). Body weight change since menopause and Percent body fat mass are predictors of subsequent bone mineral density change of the proximal femur in women aged 75 years and older: results of a 5 year prospective study. Calcified Tissue International, 75(1), 32-39.
10.1007/s00223-003-0192-4.Blanchet, C., Giguère, Y., Prud'homme, D., Dumont, M., Rousseau, F., & Dodin, S. (2002). Association of physical activity and bone: influence of vitamin D receptor genotype. Medicine and Science in Sports and Exercise, 34(1), 24-31.
10.1097/00005768-200201000-00005.Bolam, K. A., Skinner, T. L., Jenkins, D. G., Galvao, D. A., & Taaffe, D. R. (2016). The Osteogenic Effect of Impact-Loading and Resistance Exercise on Bone Mineral Density in Middle-Aged and Older Men: A Pilot Study. International Journal of Experimental and Clinical Gerontology, 62(1), 22-32.
10.1159/000435837.Cohen, J. (2013). Statistical power analysis for the behavioral sciences. Academic press.
10.4324/9780203771587.Dhanwal D. K., Dennison, E. M., Harvey, N. C., & Cooper, C. (2011). Epidemiology of hip fracture: worldwide geographic variation. Indian Journal of Orthopaedics, 45(1), 15–22.
10.4103/0019-5413.73656.Edward, W. G., Mark, A. P., & Carl, J. (2000). Physical activity, falls, and fracture among older adult: A review of the epidemiologic evidence. American Journal of Geriatric Sociology, 48(8), 883-893.
10.1111/j.1532-5415.2000.tb06884.x.Englund, U., Littbrand, H., Sondell, A., Pettersson, U., & Bucht, G. (2005). A 1-year combined weight-bearing training program is beneficial for bone mineral density and neuromuscular function in older women. Osteoporosis International, 16(9), 1117-1123.
10.1007/s00198-004-1821-0.Gianoudis, J., Bailey, C. A., Ebeling, P.R., Nowson, C. A., Sanders. K. M., Hill, K., & Daly , R. M. (2014). Effects of a targeted multimodal exercise program incorporating high-speed power training on falls and fracture risk factors in older adults: a community-based randomized controlled trial. Journal of Bone and Mineral Research, 29(1), 182-191.
10.1002/jbmr.2014.Haff, G. G., & Triplett, N. T. (Eds.). (2015). Essentials of strength training and conditioning 4th edition. Human kinetics.
Hurley, B. F., Roth, S. M. (2000). Strength Training in the Elderly Effects on Risk Factors for Age-Related Diseases. Sports Medicine, 30(4), 249-268.
10.2165/00007256-200030040-00002.Jessup, J. V., Horne, C., Vishen, R. K., & Wheeler, D. (2003). Effects of exercise on bone density, balance, and self-efficacy in older women. Biological Research for Nursing, 4(3), 171-180.
10.1177/1099800402239628.Johnell O. & Kanis, J. (2005) Epidemiology of osteoporotic fractures. Osteoporosis International, 16(2), 3–7.
10.1007/s00198-004-1702-6.Johnell, O., & Kanis, J. A. (2006). An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporosis International, 17(12), 1726-1733.
10.1007/s00198-006-0172-4.Kanders, B., Dempster, D. W., & Lindsay, R. (1988). Interaction of calcium nutrition and physical activity on bone mass in young women. Journal of Bone and Mineral Research, 3(2), 145-149.
10.1002/jbmr.5650030204.Kemmler, W., & von Stengel, S. (2013). Exercise Frequency, Health Risk Factors, and Diseases of the Elderly. Archives of Physical Medicine and Rehabilitation, 94(11), 2046-2053.
10.1016/j.apmr.2013.05.013.Kemmler, W., Haberle, L., & von Stengel, S. (2013). Effects of exercise on fracture reduction in older adults: a systematic review and meta-analysis. Osteoporosis International, 24(7), 1937-1950.
10.1007/s00198-012-2248-7.Kerr, D., Ackland, T., Maslen, B., Morton, A., & Prince, R. (2001). Resistance training over 2 years increases bone mass in calcium‐replete postmenopausal women. Journal of Bone and Mineral Research, 16(1), 175-181.
10.1359/jbmr.2001.16.1.175.Kim, C. H., & K. C. (2008). Effectiveness of a community-based exercise intervention of falls and falls risk factors for reducing risk of osteoporosis fracture. The Korean Journal of Measurement and Evaluation in Physical Education and Sports Science, 10(3), 81-90.
10.21797/ksme.2008.10.3.005.Kohrt, W. M., Bloomfield, S. A., Little, K. D., Nelson, M. E., & Yingling, V. R. (2004). Physical activity and bone health. Medicine & Science in Sports & Exercise, 36(11), 1985-1996
. 10.1249/01.MSS.0000142662.21767.58.Korpelainen, R., Keinanen-Kiukaanniemi S., Heikkinen J., Vaananen K., & Korpelainen J. (2006). Effect of exercise on extraskeletal risk factors for hip fractures in elderly women with low BMD: a population-based randomized controlled trial. Journal of Bone and Mineral Research, 21(5), 772–779.
10.1359/jbmr.060116.Langlois, J. A., Harris, T., Looker, A. C., & Madans, J. (1996). Weight change between age 50 years and old age is associated with risk of hip fracture in white women aged 67 years and older. Archives of Internal Medicine, 156(9), 989-994.
10.1001/archinte.1996.00440090089009.Liberati, A., Altman, D. G., Tetzlaff, J., Mulrow, C., Gøtzsche, P. C., Ioannidis, J. P., ... & Moher, D. (2009). The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS medicine, 6(7), e1000100.
10.1371/journal.pmed.1000100.Lipsey, M. W., & Wilson, D. B. (2001). Practical meta-analysis.
Marques, E. A., Mota, J., & Carvalho, J. (2012). Exercise effects on bone mineral density in older adults: a meta-analysis of randomized controlled trials. Age, 34(6), 1493-1515.
10.1007/s11357-011-9311-8.Marques, E. A., Mota, J., Machado, L., Sousa, F., Coelho, M, Moreira, P., & Carvalho, J. (2011). Multicomponent training program with weight-bearing exercises elicits favorable bone density, muscle strength, and balance adaptations in older women. Calcified Tissue International, 88(2), 117-129.
10.1007/s00223-010-9437-1.Marques, E. A., Wanderley, F., Machado, L., Sousa, F., Viana, J. L., Concalves, D. M., Moreira, P., Mota, J., & Carvalho, J. (2011). Effects of resistance and aerobic exercise on physical function, bone mineral density, OPG and RANKL in older women. Experimental Gerontology, 46(7), 524-532.
10.1016/j.exger.2011.02.005.Moher, D., Liberati, A., Tetzlaff, J., & Altman, D. G. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Annals of Internal Medicine, 151(4), 264-269.
10.7326/0003-4819-151-4-200908180-00135.Nair, K. S. (2005). Aging muscle. American Journal of Clinical Nutrition, 81(5), 953-963.
10.1093/ajcn/81.5.953.Nguyen, T. V., Sambrook, P. N., & Eisman, J. A. (1998). Bone loss, physical activity, and weight change in elderly women: the Dubbo Osteoporosis Epidemiology Study. Journal of Bone and Mineral Research, 13(9), 1458-1467.
10.1359/jbmr.1998.13.9.1458.Nicholson, V. P., Mckean, M. R., Slater, G. J., Kerr, A., & Burkett, B. J. (2015). Low-Load Very High-Repetition Resistance Training Attenuates Bone Loss at the Lumbar Spine in Active Post-menopausal Women. Calcified Tissue International, 96(6), 490-499.
10.1007/s00223-015-9976-6.OECD (2015). Health at a Glance 2011. OECD Indicators, OECD Publishing, Paris. DOI: https://doi.org/10.1787/health_glance-2015-en. Accessed February, 15, 2016.
10.1787/health_glance-2015-en.Park, H. M., Song, M. S., & Hur, M. (2003). A comparative study of whole body BMD with regional BMD in diagnosis of osteoporosis. Journal of Menopausal Medicine, 9(1), 25-35.
Park, H., Kim, K. J., Komatsu, T., Park, S. K., & Mutoh, Y. (2008). Effect of combined exercise training on bone, body balance, and gait ability: a randomized controlled study in community-dwelling elderly women. Journal of Bone and Mineral Metabolism, 26(3), 254-259.
10.1007/s00774-007-0819-z.Park, S. H., Shin, S. Y., & Won, D. Y. (2015). A meta-analysis of employee’s job burnout and turnover intention in sports service industry. Korean Journal of Sport Science, 26(2), 304-319.
10.24985/kjss.2015.26.2.304.Pluijm, S. M., Visser, M., Smit, J. H., Popp-Snijders, C., Roos, J. C., & Lips, P. (2001). Determinants of bone mineral density in older men and women: body composition as mediator, Journal of Bone and Mineral Research, 16(11), 2142-2151.
10.1359/jbmr.2001.16.11.2142.Protiva, K. W. (1997). Weighted vest exercise improves functional ability in women over 75 years of age. Corvallis, Oregon: Oregon State University.
Pruitt, L. A., Taaffe, D. R., & Marcus, R. (1995). Effects of a One-Year High-Intensity Versus Low-Intensity Resistance Training Program on Bone Mineral Density in Older Women. Journal of Bone and Mineral Research, 10(11), 1788-1795.
10.1002/jbmr.5650101123.Rhodes, E. C., Martin, A. D., Taunton, J. E., Donnelly, M., Warren, J., & Elliot, J. (2000). Effects of one year of resistance training on the relation between muscular strength and bone density in elderly women. British Journal of Sports Medicine, 34(1), 18-22.
10.1136/bjsm.34.1.18.Sinaki, M. (1989). Exercise and Osteoporosis. Archives of Physical Medicine and Rehabilitation, 70(3), 220-229.
Singh, M., Nagrath, A., & Maini, P. S. (1970). Changes in trabecular pattern of the upper end of the femur as an index of osteoporosis. JBJS, 52(3), 457-467.
10.2106/00004623-197052030-00005.Suominen, H. (2006). Muscle training for bone strength. Aging Clinical and Experimental Research, 18(2), 85-93.
10.1007/BF03327422.Taaffe, D. R., Duret, C., Wheeler, S., & Marcus, R. (1999). Once-weekly resistance exercise improves muscle strength and neuromuscular performance in older adults. Journal of the American Geriatrics Society, 47(10), 1208-1214.
10.1111/j.1532-5415.1999.tb05201.x.Vincent, K. R., & Braith, R. W. (2002). Resistance exercise and bone turnover in elderly men and women. Medicine and Science in Sports and Exercise, 34(1), 17-23.
10.1097/00005768-200201000-00004.Warburton, D. E. R., Nicol, C. W., & Bredin, S. S. (2006). Health benefits of physical activity: the evidence. Canadian Medical Association Journal, 174(6), 801-809.
10.1503/cmaj.051351.Whiteford, J., Ackland, T. R., Dhaliwal, S. S., James, A. P., Woodhouse, J. J., Price, R., Prince, D. A., & Kerr, D. A. (2010). Effects of a 1-year randomized controlled trial of resistance training on lower limb bone and muscle structure and function in older men. Osteoporosis International, 21(9), 1529-1536.
10.1007/s00198-009-1132-6.Woo, J., Hong, A., Lau, E., & Lynn, H. (2007). A randomized controlled trial of Tai Chi and resistance exercise on bone health, muscle strength and balance in community-living elderly people. Age and Ageing, 36(3), 262-268.
10.1093/ageing/afm005.Yi, G. S., Oh, M. K., Kim, H. G., Kang, J. A, & Kim, J, Y. (2011). Relationship between body composition and bone mineral density in elderly. Korean Journal of Clinical Geriatrics, 12(4), 160-169.