Development of an Evidence-Based Exercise Program for Childhood Cancer Survivors: A Feasibility and Pilot Study
Article information
Abstract
This study aimed to develop an exercise program for childhood cancer survivors and examine its feasibility and effects on improvements in physical fitness, muscle strength, and body composition. A tailored exercise program for childhood cancer survivors was developed through 8 systematic procedures, including a review of literature, physical activity survey, qualitative study, the first expert panel discussion, drafting an evidence-based exercise program, secondary expert panel discussion, revising the exercise program, and conducting feasibility and pilot study. For the feasibility and pilot study, 10 childhood cancer survivors (mean age 16.30 ± 1.77 years) participated, divided into either an exercise or a control group. Participants in the exercise group participated in the exercise program for 6 weeks. Based on preliminary studies, the exercise programs consisted of home-based and supervised exercise programs, including resistance and sports, conducted for 6 weeks. The body composition was measured, and a 6-minute walk test, grip strength, vertical jump, sit-up, push-up, chair stand test, and sit and reach test was conducted. After completing the exercise program, muscular endurance (sit-up test, p-value = 0.039) and lower body strength (chair stand test, p-value = 0.010) were significantly increased in the exercise group compared to the control group. Fat mass significantly decreased in the exercise group compared to the control group (p-value = 0.010). In conclusion, the exercise program developed in this study demonstrated feasibility and effectiveness in reducing body fat mass and improving muscular endurance and lower body strength in childhood cancer survivors.
Introduction
Childhood cancer is the leading cause of death among children and adolescents (Bhakta et al., 2019; Force et al., 2019). Thus, advances in cancer treatment and early detection are significantly important for childhood cancer survivorship (Howlader et al., 2017). Childhood cancer treatment can result in various adverse physiological and psychosocial problems for survivors (Nightingale et al., 2011). Moreover, cancer treatment can increase the risk of late effects, including well-established dose-response associations between anthracycline chemotherapy, chest-directed radiation, and the risk of cardiomyopathy and cardiac-specific mortality (Mulrooney et al., 2016).
Increasing physical activity (PA) and maintaining physical fitness are key preventive actions against childhood cancer. A meta-analysis and review study (Morales et al., 2018) suggested that both (or almost any type of exercise) aerobic and resistance exercises can improve functional mobility in children with any cancer, while exercise training during cancer treatment seemed not to increase the risk of cancer-specific mortality, recurrence, or other adverse outcomes. Cardiorespiratory fitness (CRF) and muscle strength are reduced during and after childhood cancer treatment (De Caro et al., 2006; van Brussel et al., 2006). Physical inactivity may cause reduced physical fitness among childhood cancer survivors (Fuemmeler et al., 2013). Additionally, overweight and obesity are more prevalent among childhood cancer survivors, with leukemia survivors having the highest rates (Meacham et al., 2005; Oeffinger et al., 2003; Zhang & Parsons, 2015).
Participating in regular PA and exercise is advantageous for improving the prognosis of childhood cancer. Previous studies have found that exercise is feasible and safe for childhood cancer survivors without adverse effects (Baumann et al., 2013; Huang & Ness, 2011; San Juan et al., 2010). Although there is limited evidence regarding the beneficial role of PA in improving childhood cancer outcomes across different levels (severity) of childhood cancer, strong evidence has shown that exercise training has positive effects on any type of childhood cancer and its related outcomes (Morales et al., 2018). PA can mitigate or prevent late effects, including pain, fatigue, obesity, diabetes, and cardiovascular disease in childhood cancer survivors (Alvarez et al., 2007; Diller et al., 2009; Oeffinger et al., 2006). In contrast, PA can improve overall health, functional capacity, quality of life (QOL), fitness, muscular strength, fatigue, and mental health in childhood cancer survivors (Keats & Culos-Reed, 2008; San Juan et al., 2007, 2010). Furthermore, Scott et al. (2018) reported that an increased exercise volume (7.9±4.4 metabolic equivalent task (MET)-h/wk) over 8 years was associated with a 40% lower risk of all-cause mortality rate compared to the lower exercise level. Especially, vigorous exercise was associated with a lower prevalence of depression and somatization, even in childhood cancer survivors grew up. Among childhood cancer survivors who were adherent to national guidelines for vigorous activity (≥9 MET-hours per week), compared with survivors who did not meet guidelines, the adjusted prevalence ratio for those with depression was 0.80 (95% CI, 0.68–0.94; p = 0.009), and the adjusted prevalence ratio for those with somatization was 0.83 (95% CI, 0.72–0.94; p = 0.001) (Tonorezos et al., 2019).
Although regular PA and exercise can provide significant health benefits for childhood cancer survivors, evidence-based exercise programs were limited, considering their characteristics. Due to the differences between childhood cancer survivors and the general population, as well as differences among childhood cancer survivors based on factors such as cancer type, age at diagnosis, and treatment received, it is important to develop an exercise program tailored specifically for childhood cancer survivors to maintain optimal health status. Furthermore, there is currently a lack of exercise programs tailored to childhood cancer survivors’ unique needs and preferences. Therefore, this study aims to develop and evaluate the feasibility and efficacy of an exercise program specifically designed for childhood cancer survivors, focusing on improving physical fitness and body composition.
Methods
Development of an Evidence-Based Exercise Program for Childhood Cancer Survivors
An exercise program for childhood cancer survivors was developed through 8 systematic procedures, including (1) a literature review, (2) a PA survey, (3) a qualitative study, (4) the first expert panel discussion, (5) drafting an evidence-based exercise program, (6) secondary expert panel discussion, (7) revising the exercise program, and (8) feasibility and pilot study (Figure 1).
PA survey was conducted on 120 childhood cancer survivors (72 boys, 48 girls) aged 8–18 years from a pediatric oncology center in Seoul, South Korea, between March and August 2017 using a previously validated PA questionnaire for childhood and cancer (Kim et al., 2022a). The survey aimed to identify the participation, preferences, and barriers to PA in childhood cancer survivors. The survey results showed that the top 5 preferred exercise activities were soccer (25.8%), basketball (23.3%), resistance exercise (22.5%), badminton (19.2%), and dance (16.7%). In addition, 65 participants (54.2%) preferred exercising with friends, and 57 participants (47.5%) did not have a specific exercise location preference. Moreover, 67 participants (55.8%) chose to exercise for more than 30 minutes, and 75 participants (62.5%) exercised between 2 and 3 times per week.
Qualitative research was conducted using a previously validated PA barriers questionnaire for childhood cancer survivors (Kim et al., 2022b) to identify the exercise participation experience and determine barriers to PA. This in-depth study used semi-structured interviews that utilized open-ended questions. The results showed that before cancer diagnosis, the study participants experienced similar exercise types and PA participation as children and adolescents without cancer. However, during their cancer treatment, their PA participation dramatically decreased, and even after the completion of cancer treatment, participants did not regain their pre-diagnosis PA levels. The participants experienced barriers to exercise, classified into ‘environmental constraint factors’, such as lack of exercise partners and facilities, and ‘physical constraint factors’, such as low fitness levels and side effects of cancer treatment.
After completing the survey and interview, the first expert panel discussion was conducted. Experts from childhood cancer, family medicine, and sports were invited to discuss the survey and qualitative study results and provide their opinions on the development of an exercise program. The input of the expert panel was included while drafting the exercise program. An exercise program was developed based on the literature review, survey and qualitative research results, and expert opinions. The exercise program included soccer, baseball, resistance exercise, badminton, and dance for an hour twice a week, according to the exercise preferences.
Second expert panel discussions were held with experts in childhood cancer, family medicine, and sports to refine the exercise program. The exercise program draft was revised according to the experts’ feedback. The exercise program for childhood cancer survivors was finalized through surveys, in-depth studies, and expert opinions. The exercise intensity and duration were adjusted differently based on the unique characteristics of each cancer type, and 3 assistant exercise instructors monitored participants during exercise sessions. Lastly, a pilot study was conducted to examine the feasibility and effectiveness of the developed exercise program.
Study Design and Participants
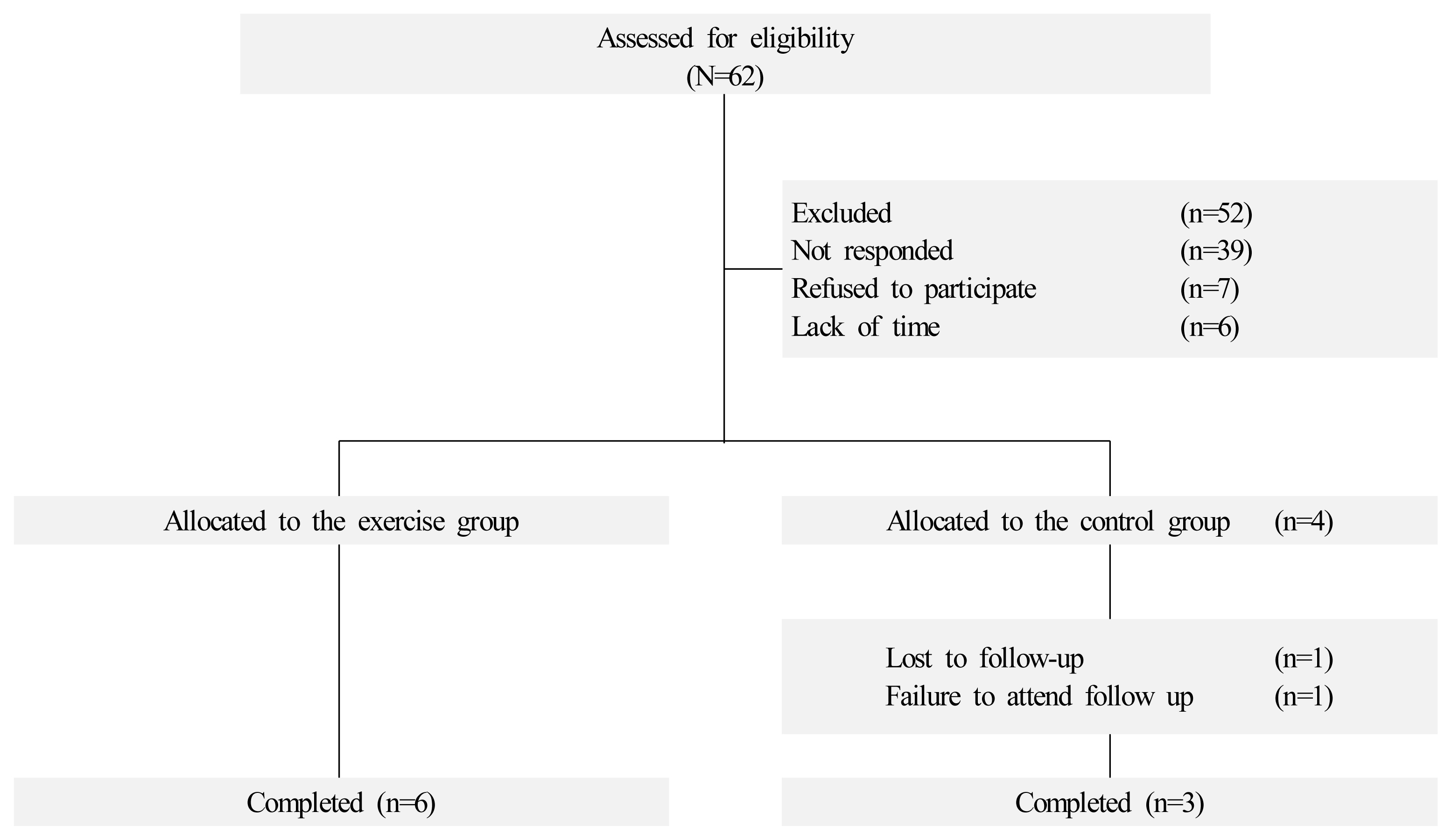
Children and adolescents diagnosed with cancer were recruited from the Yonsei Cancer Hospital between December 2017 and April 2018. The eligible criteria included childhood cancer survivors aged between 8 and 18 years who were at least 2 years post-completion of all standard surgeries and chemotherapy. Participants with severe physical abnormalities, cognitive or mental disorders were excluded. Of the 62 childhood cancer survivors approached, 52 were not interested in participating in the study, and a total of 10 childhood cancer survivors participated in the study. A quasi-experimental study was conducted, with 6 participants assigned to the exercise group and 4 participants to the control group (Figure 2).
Before participating in the study, all participants and their parents signed a consent form. The study was approved by the institutional review board at the Yonsei University College of Medicine (IRB no. 4-2017-1012).
Measurements
The personal information, including patient demographic information, cancer type, treatment history, and medical history, was collected using medical records. During the anthropometric assessments, participants were asked to wear light clothing with bare feet. Height was measured using a stadiometer, rounding up to the nearest 0.1, and body weight was measured using a general scale, rounding up to the nearest 0.1 kg. In addition, body composition was measured using a bioelectrical-impedance analysis measure (In-body 720, Biospace, Korea).
The 6-minute walk test was conducted to assess cardiopulmonary fitness (Li et al., 2005). Participants were instructed to walk as fast as possible for 6 minutes, and the distance was recorded in meters. The hand grip strength test was conducted to assess muscular strength (Hager-Ross & Rosblad, 2002) using a handheld dynamometer. Participants were tested while they were standing with their arms extended. The vertical jump test was used to measure the lower body muscle power. Participants were instructed to jump vertically as high as possible, using their arms and legs to assist in projecting the body upwards. The sit-up test was conducted to assess muscular endurance.
Participants laid on a mat with their knees bent and raised their chests as much as possible every 3 seconds without a time limit. In addition, the push-up test was also conducted to assess muscular endurance (Pate et al., 1993). The participants were instructed to perform push-ups with their hands and toes touching the floor while keeping their bodies and legs straight. Female participants performed the push-ups with bent knees. The chair stand test was used to assess lower body strength, counting the number of full stands completed in 30 seconds with arms folded in front of the chest. The sit and reach test was used to assess flexibility. Participants sat on the mat with their legs extended and hands reaching toward their toes. All feasibility and pilot study measurements were performed at baseline and after 6 weeks.
Exercise Program
The exercise programs consisted of home-based and supervised exercise programs conducted for 6 weeks based on their exercise preferences (Kim et al., 2022a). Before starting the exercise program, exercise experts discussed the type of exercise, intensity, and duration with physicians. Participants in the exercise group conducted both home-based and supervised exercise programs.
The objective of the home-based exercise program was to increase the level of PA. The participants in the exercise group were provided with an exercise diary, including a daily activity log. After each session’s completion, they recorded their activity’s type and duration. Participants were expected to participate in a daily resistance exercise program of upper, lower, and muscular endurance with 3 difficulty levels. The researcher checked the exercise diary once a week to monitor adherence to the exercise program.
Additionally, 12 supervised exercise program sessions were conducted twice a week for 6 weeks with exercise experts as a form of group exercise (Table 1). Each session of the supervised exercise program included warm-up (5~15 min), line dance (15 min), resistance (20~30 min), and sports (40 min) such as basketball, badminton, soccer, and racket sports considering their preferences (Kim et al., 2022a). For resistance exercise, the intensity was adjusted to individual physical fitness, moderate or vigorous. The sports sessions were conducted individually to their physical function and experience for the sports. Exercise experts instructed the participants on sports and exercised together. The type of exercise can be defined as combined (aerobic-based, resistance training-based, sport activity-based), and the total duration of each exercise session was 80~100 min.
Data Analysis
The analysis was conducted based on an intent-to-treat (ITT) principle, with the last observation carried forward to account for missing data. Statistical analyses were performed using the Statistical Package for Predictive Analytics SoftWare (PASW) SPSS, version 23.0 (SPSS Inc, Chicago, IL, USA). Normality was examined using the Shapiro-Wilk test, and the differences in categorical variables between the exercise and control groups were assessed using the Chi-Square test. The differences in physical fitness, muscular strength, and body composition between baseline and post-intervention were examined using the Wilcoxon-Signed Ranks test. The differences in the changes of study variables between the exercise and control groups were analyzed using the Mann-Whitney test. A p-value < 0.05 was considered statistically significant.
Results
Baseline Characteristics
Ten childhood cancer survivors were recruited for the study, and baseline characteristics were not different between the exercise and control groups (Table 2). The mean age of the participants was 16.30 ± 1.77 years, and the mean age of cancer diagnosis was 10.40 ± 4.50 years. One participant in the control group dropped out of the study due to failure to attend follow-up.
Feasibility of the Exercise Program for Childhood Cancer Survivors
All 6 participants enrolled in the exercise group completed the study. Table 3 shows the adherence rate to the exercise program of participants in the exercise group. The average of adherence rate was 93.05%.
Effects of the Exercise Program on Physical Fitness and Body Composition
Table 4 presents the physical fitness between the exercise and control groups at baseline and after the 6-week exercise program. The sit-up test (pre: 27.50 ± 7.87 reps, post: 41.67 ± 6.43 reps, p-value = 0.039) and the chair stand test (pre: 15.67 ± 2.94 reps, post: 19.17 ± 3.31 reps, p-value = 0.010) were significantly improved after the 6 weeks of intervention in the exercise group only. Also, the changes in the sit-up and chair stand tests in the exercise groups were significantly greater than in the control group (all p-value < 0.05).
Table 5 presents the body composition between the exercise and control groups at baseline and after the 6-week exercise program. Body fat mass was significantly reduced (pre: 21.93 ± 11.43 kg, post: 21.17 ± 11.24 kg, p-value=0.008) after the 6 weeks of intervention in the exercise group only. Also, the reduction of body fat mass was significantly greater than that of the control group (p-value = 0.010).
Discussion
The purpose of the study was to develop an evidence-based exercise program for childhood cancer survivors and to examine the feasibility and effects of the exercise program. The evidence-based exercise program was developed through 8 systematic procedures: a literature review, PA survey, qualitative study, 1st expert discussion draft exercise program, 2nd expert discussion, and revised program. The final procedure was to confirm the safety and efficacy of the exercise program with a feasibility and pilot study.
In the feasibility and pilot study, the average adherence rate in the exercise group was 93.08%. In addition, there were no dropouts or injuries in the exercise group. Constructing the exercise program for childhood cancer survivors, discussions on safety should be preceded(Antwi et al., 2019). Since the exercise intensity of this exercise program was adjusted according to the participant’s medical records and personal characteristics and experts in each field predicted and continuously discussed possible side effects or injuries from participating in the exercise program, there were no side effects or injuries. Moreover, the high rate of exercise participation in this study could be interpreted as a result of providing a tailored exercise program developed through expert consultation and the survey, with consideration for exercise barriers and preferences. It can be seen that they have increased their interest by operating the sports event they want as an exercise program. In the case of children and adolescents, continuous motivation and fun factors are reported to be very important in increasing exercise participation(Kudlacek et al., 2020). Since the exercise adherence rate is directly related to the exercise effect, providing an exercise program that accommodates the participant’s characteristics is crucial.
In addition, the participants in the exercise group improved their physical fitness and body composition after completing the exercise program. In particular, muscular endurance (sit-up test, p-value = 0.039) and lower body strength (chair stand test, p-value = 0.010) significantly increased. At the same time, fat mass significantly decreased in the exercise group compared to the control group. These results are inferred to be due to the high rate of exercise participation and increased overall physical activity levels of the exercise group. However, muscular strength, body weight, muscle mass, and waist circumferences in the exercise group were not changed compared to the control group. It was expected that the exercise program’s volume (intensity, duration, time, frequency) might not be full to show any changes in muscular strength tests and other components of body composition. Therefore, additional research of randomized controlled trials with larger samples or longer study durations will be needed to examine the effects of the exercise program on physical fitness, muscular endurance, and body composition in childhood cancer survivors.
Adolescents’ physical and psychological health is significantly linked to the late effect on their adult age(Hallal et al., 2006; Otto et al., 2021). In addition, it is reported that children and adolescent health care will play an essential role in reducing socioeconomic costs at the individual and national levels in adulthood (Armocida et al., 2022; Jekal et al., 2014; Kassebaum et al., 2017). Most of the previous studies of exercise programs for childhood cancer survivors have verified the effects on quality of life, fatigue, cognitive function, bone health, and physical activity levels (Bernal et al., 2022; Beulertz et al., 2016; Jung et al., 2023; Le et al., 2017; Li at al., 2018). This study has value as a preliminary study that developed a feasible and effective exercise program for childhood cancer survivors and verified the improvement of physical fitness and body composition in childhood cancer survivors by participating in an exercise program. Considering the improvement in the 5-year relative survival of childhood cancer in Korea (56.7% from 1993 to 1995, 85.8% from 2015 to 2019) and the increase in the number of patients with cancer, large-scale multicenter clinical studies are continuously needed to improve the outcome of childhood cancer survivors (Ministry of Health and Welfare, 2021). Since childhood cancer survivors could gain health benefits from regular exercise, motivating them to exercise more is essential (Kim et al., 2022a).
There are several limitations. First, given the small sample size of this study, the study findings may not be generalized to wider populations. Second, there could be some residual confounding factors, such as dietary information, energy intake, and medication intake. In addition, given the quasi-experimental study design, the study findings cannot suggest sufficient causal relationships between study variables. Thus, additional research of randomized controlled trials with larger samples or longer study durations is needed to examine better the effects of the exercise program on physical fitness, muscular endurance, and body composition in childhood cancer survivors.
In conclusion, this study developed and verified the evidence-based exercise program procedure for childhood cancer survivors. This exercise program was developed by examining actual childhood cancer survivors’ exercise preferences and barriers and discussing them with experts in each field. At the feasibility step, the safety and practicality of this exercise program were confirmed as a pilot study. Furthermore, the stage of this evidence-based exercise program could be a valuable tool for fitness specialists, healthcare professionals, and medical and health services to adapt exercise programs to their needs.
Acknowledgments
This work was supported by Korean Foundation for Cancer Research (KFCR-2017-001).