Effects of Exercise Intensity during the Detraining Period on Changed Muscle Hypertrophy- and Mitochondrial Biogenesis-Related Proteins after High-Intensity Interval Training
Article information
Abstract
This study investigated whether different exercise intensities during detraining could maintain muscle hypertrophy and mitochondrial biogenesis signaling pathways activated high-intensity interval training (HIIT) in the flexor pollicis longus and soleus muscles of Sprague-Dawley rats. Fiver groups were studied: non-exercise ([NEG], n=6), HIIT only ([CTG], n=6), resting control ([CD], n=6), low-intensity exercise (LID, n=6), moderate-intensity exercise ([MID], n=6). The HIIT program consisted of 30-minute sessions of 24 m/min for eight weeks. During detraining (two weeks), LID and MID groups exercise three times per week at 8 m/min and 16 m/min, respectively. Muscle hypertrophy and mitochondrial biogenesis-related protein expression in the flexor pollicis longus and soleus muscles were analyzed using Western blotting. Compared to NEG, PI3K was higher in the CTG and LID in the flexor pollicis longus, while Akt and p-mTOR signaling pathways were significantly activated in MID and CTG. In the soleus muscle, p-AMPK expression was higher in CD and MID than in NEG, and FNDC5 was upregulated in LID compared to CTG and CD. These findings suggest that moderate-intensity exercise during detraining may help maintain HIIT-induced muscle hypertrophy signaling, while low-intensity exercise may contribute to sustaining HIIT-activated aerobic metabolism in skeletal muscle.
Introduction
It has been well documented that skeletal muscle in the human body is sensitively changed according to regular training or short- and long-term detraining (Mujika & Padilla, 2000a). In the field of physical training, the FITT (frequency, intensity, exercise type and rest time) principle of exercise regulates an adaptation of molecular, histological, and functional characteristics in skeletal muscle fibers, resulting in muscle hypertrophy or atrophy (Phillips, 2009). This adaptation of skeletal muscle is closely associated with accelerating specific protein signalling pathway (Glass, 2003; Goldspink, 2003; Gunasekera et al., 2003), and it is necessary to understand the metabolic adaptation occurring in skeletal muscles.
In general, various previous studies have been reported that resistance exercise improves skeletal muscle hypertrophy and function by activation of Phosphoinositide 3-kinases (PI3K)/ Protein kinase B (Akt)/ mechanistic target of rapamycin (mTOR), mitogen-activated protein kinase (MAPK) and Ca2+/calmodulin-dependent protein kinase (CaMK) signalling pathways (Goldspink, 2003). Phosphorylation of Akt-mTOR promotes differentiation in satellite cells located in the skeletal muscle (Glass, 2003). Also, upregulation in insulin-like growth factor (IGF-1), an upstream molecule of Akt, after regular high-intensity interval training (HIIT) facilitates the rate of protein synthesis in skeletal muscle for hypertrophy and/or hyperplasia (Yang & Goldspink, 2002).
Aerobic exercise is effective in reducing body weight through promoting fat oxidation, and this phenomenon occurs due to an increase in mitochondrial biogenesis regulatory cascade such as Peroxisome proliferator-activated receptor-gamma coactivator-1 alpha (PGC1-α) (Liang & Ward, 2006). PGC1-α is a downstream protein of adenosine mono-phosphate-activated protein kinase (AMPK). Regular aerobic exercise accelerates the AMPK-PGC1-α signalling pathway to positively regulate mitochondrial biogenesis in the skeletal muscle (Coffey et al., 2007)
Considering previous studies related to skeletal muscle adaptation to exercise, performing regular aerobic and/or anaerobic exercise increased skeletal muscle hypertrophy and activated energy metabolism. But modern people require a special strategy to maintain the effects of prior exercise for a long time after the period of training cessation (Glass, 2003; Goldspink, 2003).
Detraining is defined as phenomenon in which enhanced physiological and physical elements (VO2max, glycogen storage and lactate threshold) after long-term training return to the pre-exercise state (Mujika & Padilla, 2000b). In previous studies on the changes in physiological characteristics during detraining, Sheibani et al. (2020) found that the weight and size of the soleus muscle and myocardium were decreased within 7 days after stopping training, and activation of FoxO3a and MAFbx transcription factors prevented proliferation and hypertrophy in cardiomyocytes. Omidi & Yousefi (2019) reported that the improvement of fasting blood glucose, insulin sensitivity and glycated haemoglobin in diabetic rats after aerobic treadmill exercise rapidly went back to the pre-exercise state within 4 weeks after stopping exercise.
Taken together, previous studies have only focused on exercise type and frequency on physiological variables during detraining (Applegate et al., 1984; Radak et al., 2006). Reliable studies on role of the exercise intensity and intramuscular signalling metabolism during the period of detraining are lacking. Therefore, the purpose of this study is to confirm whether different exercise intensities during the detraining period can maintain HIIT-induced muscle hypertrophy- and mitochondrial biogenesis-related signalling pathway in the flexor pollicis longus and soleus muscles of the rats.
Methods
Experimental Animals
The male Sprague-Dawley (SD) rats (5 weeks old, N=30) were used in this experiment. They were randomly divided into five groups; non-exercise group (NEG, n=6), the group that completed HIIT (CTG, n=6), the resting control group (CD, n=6), the low-intensity exercise group (LID, n=6), and the moderate-intensity exercise group (MID, n=6) during the detraining period. They were accepted to eat commercial rat chow (Samyang Co., Seoul, Korea) and water ad libitum. All rats were maintained constant room temperature of 22°C and 60% of humidity under 12/12-hr light-dark cycle at Jeju national university laboratory animal centre for 10 weeks. This study was approved by the Jeju National University Institutional Review Board (2022-0026).
Exercise Protocol
Before starting the study, all rats underwent an exercise adaptation (at a speed of 10~24m/min) during 1 week. For HIIT, treadmill exercise was performed in 10 sets at a speed of 24m/min for 20 min with no inclination at 3 times per week for 8 weeks. Training program on HIIT consisted of maximum running for 1 min and complete rest for 1 min. During 2-week detraining period, the low-intensity exercise group reduced 60% from high-intensity exercise intensity to 8m/min, and the moderate-intensity exercise group reduced 30% from high-intensity exercise intensity to 16m/min. Treadmill exercise was performed 3 times a week for 30 min, and the complete rest group did not apply any other exercise (Wisloff et al., 2001).
Western Blot Analysis
All rats were sacrificed 48 hours after the end of exercise. The dissected flexor pollicis longus and soleus muscles tissues were harvested and rinsed with phosphate-buffer saline and lysed in Triton lysis buffer. Denatured proteins were separated on sodium dodecyl sulphate-polyacrylamide gel and then transferred onto polyvinylidene difluoride membrane on ice at 200mA for 2 hours. The membranes were blocked with 5% BSA and washed with 0.1% Tween 20 in tris buffered saline for 30 min at room temperature. The membranes were incubated overnight at 4°C with primary antibodies.
Protein (20μg) was used for Western blot analysis using anti-PI3K mouse polyclonal antibody (1:1,000, Cell Signalling Bio-technology, Danvers, MA, USA), anti-phosphorylated mTOR rabbit polyclonal antibody (1:1,000, Cell Signalling) anti-phosphorylated Akt rabbit polyclonal antibody (1:1,000, Cell Signalling), anti-phosphorylated ERK1/2 rabbit monoclonal antibody (1:1,000, Cell Signalling), anti-GAPDH mouse monoclonal antibody (1:1,000, Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-phosphorylated AMPK mouse polyclonal antibody (1:1,000, Cell Signalling), anti-PGC-1α mouse monoclonal antibody (1:1,000, Cell Signalling), anti-FNDC5 mouse polyclonal antibody (1:1,000, Cell Signalling). For the secondary antibody, Horseradish peroxidase-conjugated anti-mouse or rabbit IgG antibodies (1:1,000, GeneTex Inc., Irvine, CA, USA) were used. The blotting proteins were detected using Westar ECL substrates (Cyanagen, Bologna, Italy). And, detected protein band density was analysed using Chemidoc (Bio-Rad, Hercules, CA, USA).
Statistics
All the data were presented as mean ± standard. Statistical analysis was performed using one-way ANOVA of variance followed by Duncan post hoc test. The significance level was set at p <0.05. All data and graphs were performed using Prism 6 (GraphPad, La Jolla, CA, USA).
Results
Muscle Hypertrophy Signalling Pathway in Flexor Pollicis Longus Muscle
To examine expression level in hypertrophy-related proteins according to exercise intensity during detraining period, flexor pollicis longus muscle was prepared and used in Western blot analysis. As shown in Figure 2, PI3K (F=6.020, p=.010) was significantly increased in the CTG and LID compared to the NEG. However, there was no significant difference between groups in p-ERK1/2 (F=1.435, p=.292). p-Akt (F=32.091, p=.001) was significantly upregulated in the MID and CD compared to the NEG, CTG and LID. Phospho-mTOR (F=5.053, p=.017) was significantly increased in the CTG, MID compared to the NEG, CD and LID.

Muscle hypertrophy signalling pathway in flexor pollicis longus muscle, p <.05 vs NEG; a, p <.05 vs CTG; b, p <.05 vs CD; c, p <.05 vs LID; d, non-exercise group (NEG), the group that completed HIIT (CTG), the resting group (CD), the low-intensity exercise group (LID), and the moderate-intensity exercise group (MID)
Mitochondrial Biogenesis Signalling Pathway in Soleus Muscle
To examine expression level in mitochondrial biogenesis-related proteins according to exercise intensity during detraining period, soleus muscle was prepared and used in Western blot analysis. As shown in Figure 3, p-AMPK (F=5.053, p=.017) was significantly increased in the CD and MID compared to the NEG. However, PGC1-α (F=3.267, p=.059) was not significant difference in all groups. And FNDC5 (F=31.310, p=.001) was significantly upregulated in the LID compared to all groups, and the CTG and CD showed higher expression levels than the NEG.
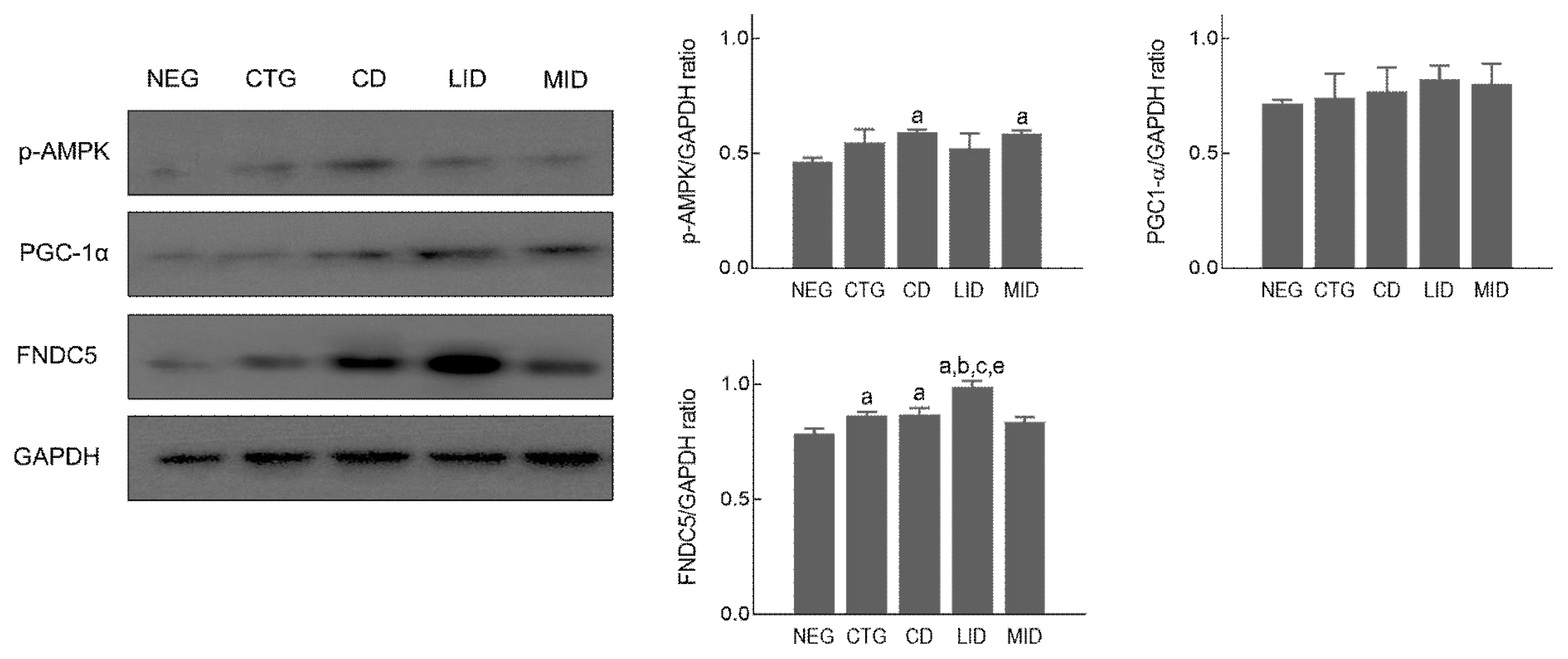
Mitochondrial biogenesis signalling pathway in soleus muscle, p <.05 vs NEG; a, p <.05 vs CTG; b, p <.05 vs CD; c, p <.05 vs MID; e, non-exercise group (NEG), the group that completed HIIT (CTG), the resting group (CD), the low-intensity exercise group (LID), and the moderate-intensity exercise group (MID)
Discussion
In the present study, we found out the appropriate exercise intensity to maintain HIIT-improved muscle hypertrophy- and mitochondrial biogenesis-related signalling pathway in the flexor pollicis longus and soleus muscles of rats.
The flexor pollicis longus muscle is one of three deep muscles located on the back of rat forelimb, and this muscle is attached to the plantar surface of the distal phalanx of the great toe to induce plantar flexion of the ankle, which has a high distribution of fast-twitch fibers with fast and strong muscle contraction and a high rate of lactate accumulation. Therefore, the use of flexor pollicis longus muscle in the present study is suitable to identify the hypertrophy-related Akt-mTOR signalling pathway (Pan et al., 2016).
The soleus muscle is a muscle that extends from the lower part of the knee to the calcaneus, and slow twitch fibers are highly distributed to induce plantar flexion of the ankle (Olewnik et al., 2020). Thus, we extracted the soleus muscle for aerobic metabolism and mitochondrial biogenesis-related signalling pathways.
Previous studies reporting the mechanism of muscle hypertrophy after resistance exercise emphasized the importance of phosphorylation of PI3K, Akt, and mTOR cascades (Fukada & Ito, 2021). In addition, mTOR-overexpressing transgenic mouse could accelerate hypertrophy of skeletal muscle compared to normal rats (Bamman et al., 2018). And recent studies demonstrated that PI3K-Akt-mTOR signalling upregulated protein synthesis in skeletal muscle after resistance exercise through activation of p70S6K and 4E-BP1 (Sartori et al., 2021). In the present study, we investigated the flexor pollicis longus muscle to confirm activation of muscle hypertrophy-related PI3K-Akt-mTOR signalling pathway after 8-week high intensity training or during detraining period. As a result, the expression level of PI3K was significantly increased in the flexor pollicis longus muscle after 8 weeks of HIIT, but no statistical change was observed in the non-exercise and low-intensity exercise groups for detraining period. Activated Akt and mTOR after HIIT were rapidly downregulated in the non-exercise and low-intensity exercise groups during detraining period, whereas moderate-intensity exercise during detraining period considerably stimulated Akt and mTOR phosphorylation than non-exercise group after HIIT. Looking at previous studies analysing protein metabolism related to muscle hypertrophy, high-intensity interval training was increased the expression of Akt, PI3K, and mTOR proteins to induce gastrocnemius hypertrophy (Biglari et al., 2020), but myostatin and FoxO expression were suppressed, meaning that HIIT for a long time and moderate-intensity exercise during detraining period can increase proliferation of satellite cells for muscle hypertrophy and hyperplasia through activation of IGF-1, Akt, and mTOR signalling pathways (Liao et al., 2015). These previous findings reverified the role of Akt-mTOR signalling pathway in muscle hypertrophy, and it seems to be partially consistent with the results of this study. However, in the present study, HIIT-activated PI3K did not show significant changes in low-intensity and moderate-intensity exercise during detraining period, this information is somewhat contrary to the results of previous studies. And this phenomenon is thought to be that the period of low and moderate-intensity exercise performed during detraining was not sufficient to affect the change in PI3K expression level.
ERK1/2 is protein-serine/threonine kinases that regulate the Ras-Raf-MEK-ERK signalling transduction pathway, and this protein stimulates various cell biological processes such as proliferation, differentiation, migration, and survival of satellite cell in skeletal muscle after regular resistance exercise (Busca et al., 2016; Chen et al., 2020). In our study, ERK1/2 was significantly phosphorylated 8 weeks after HIIT, but no significant change was observed in non-exercise, low and moderate-intensity exercise groups detraining period. Several previous studies reported that moderate-intensity resistance exercise (>65% 1RM) during detraining period might lead to activation of MAPK-ERK1/2 transduction cascade for muscle hypertrophy (Seok, 2018; Taylor et al., 2012; Widegren et al., 2001). These previous studies show contradictory results to data of this study. Therefore, in future studies, it is necessary to investigate how the upstream and downstream molecules that crosstalk to p-ERK1/2 can be regulated depending on the exercise intensity during detraining period.
Aerobic physical exercise can prevent and treat obesity by increasing the number and density of mitochondria in skeletal muscle and facilitating aerobic energy metabolism (Stanford et al., 2015; Thirupathi et al., 2019). In the field of biochemistry, PGC1-α plays a role in regulating mitochondrial biogenesis in skeletal muscle, and AMPK is a major regulator of glucose metabolism and homeostasis during exercise (McConell et al., 2020). Also, FNDC5 (Irisin) is known to be an important factor in determining the amount of brown fat conversion in the body. In the present study, PGC1-α and AMPK after HIIT didn’t show no significant difference among all groups, and the low-intensity and moderate-intensity exercise during detraining period didn’t increase expression level of these proteins in the soleus muscle. In general, the increase in the number of mitochondria in skeletal muscle and enhancement in aerobic metabolism through activation of PGC1-α and AMPK is promoted when low-intensity exercise is performed for a long time (McConell et al., 2020; Rothschild et al., 2022). But our finding is thought to have contradictory results because high-intensity exercise was applied. Furthermore, FNDC5, and regulator of brown fat conversion, was not upregulated after 8-week high-intensity exercise, but low-intensity exercise during detraining period significantly increased FNDC5 induction level in soleus muscle. Xiong et al. (2019) presented that FNDC5 knockout mouse have severe obesity due to reduced brown fat conversion and Fain et al. (2013) suggested that aerobic exercise might increase FNDC5 level to decrease body weight of pigs. These previous studies related to FNDC5 are consistent with our findings.
Our findings emphasize the necessity of regular exercise during the detraining phase to preserve physiological characteristics and physical fitness improvements achieved through HIIT. Specifically, it suggests scientific evidence that the continued moderate-intensity exercise during the detraining period might maintain HIIT-activated hypertrophy- and mitochondrial biogenesis-related signalling pathway in the skeletal muscle.
Notes
Author Contribution
Conceptualization: Tae-Beom Seo
Data curation: Joo-In Yu
Formal analysis: Yeong-Hyun Cho
Methodology: Tae-Beom Seo
Projectadministration: Tae-Beom Seo
Visualization: Yeong-Hyun Cho
Writing-original draft: Joo-In Yu,
Writing-review&editing: Tea-Beom Seo
