The Impact of Whole-Body Vibration Training on Lower-Extremity Muscle Function in Older Adults: A Systematic Review and Meta-Analysis
Article information
Abstract
This review aimed to analyze the impact of whole-body-vibration (WBV) training on strength, power, muscle endurance, functional lower-extremity flexibility (FLEF), and functional lower-extremity strength (FLES) in older adults and to evaluate the effectiveness of different WBV training periods. Electronic searches were conducted using four databases. The methodological quality and level of evidence of the selected articles were assessed by two reviewers. Standardized mean differences (SMD) and 95% confidence intervals (CIs) were calculated. Nineteen studies were included (474 participants; mean age 74.27 years). Strength was measured by isokinetic, isometric, or isotonic contraction using an isokinetic dynamometer or weight-training machines. Power was measured by an assessment task that required maximal force in a short period of time. Endurance was evaluated by maximal repetition of tasks, FLEF by chair stand-and-reach and functional reach tests, and FLES by the sit-to-stand test. Meta-analysis showed that WBV training had significant effects on strength (SMD = .54; CIs = .40, .67), power (SMD = .58; CIs = .28, .89), muscle endurance (SMD = .82; CIs = .36, 1.29), FLEF (SMD = .31; CIs = .06, .55), and FLES (SMD = −.75; CIs = −1.18, −.33). Our findings suggest that 1) WBV training improves overall lower-extremity muscle function in older adults, especially muscle endurance; 2) a minimum of 8 weeks of WBV training is recommended to improve strength, muscular endurance, and power; and 3) a longer period of the WBV training is more effective at improving muscle endurance, power, and strength but not FLEF and FLES. Therefore, WBV training is strongly recommended to improve various muscle function parameters in older adults.
Introduction
Aging causes various changes in the body, such as a reduction in muscle mass and changes in the ratio of muscle fibers, resulting in decreased muscle function (Fielding et al., 2011; Goudarzian, Ghavi, et al., 2017; Kennis et al., 2013). With aging, the body movements of older adults become slower as a result of decreased physical fitness (Coelho & Araújo, 2000; Milanović et al., 2013; Visser et al., 2005). Consequently, even the most necessary physical activities in daily life, such as walking and climbing stairs, are compromised (Kennis et al., 2013). Additionally, the risk of falls, one of the leading causes of death in older adults, increases in these individuals (Landi et al., 2012; Tinetti et al., 1988). Even if a fall does not lead to death, a fall impairs the functional movements of older adults and interferes with their daily life (Li et al., 2003; Mangione & Palombaro, 2005). Changes in physical functioning have various impacts on older adults, including difficulty in maintaining an independent daily life, reluctance to engage in physical activities, and an increased risk of contracting various diseases (Erikssen, 2001; Kennis et al., 2013). Consequently, decreased physical activity in older adults results in social isolation, reduced life satisfaction (Emilio et al., 2014; Fabre et al., 2007; Vieira et al., 2013), and other psychological problems such as anxiety and depression (Means et al., 2003; Tinetti, 2003). Therefore, studies on various exercise programs for older adults to slow down muscle loss and maintain physical function have been actively conducted. Resistance and aerobic training, or a combination of both, are representative exercise programs for preventing sarcopenia and atrophy in older adults (Ferrari et al., 2016; Kanegusuku et al., 2015; Liberman et al., 2017).
The aforementioned exercise training programs have certain limitations. For example, resistance training poses an injury risk to older adults with reduced physical abilities owing to the use of weights and equipment (Frontera et al., 1988; Keogh & Winwood, 2017; Kubo et al., 2003). Aerobic training can also cause sustained load on the joints, increasing the risk of injury and causing cardiovascular problems in older adults (Mandic et al., 2012). In particular, the risk of injury increases in older adults who do not regularly participate in physical activities. To prevent the side-effects of resistance and aerobic exercises, whole-body-vibration (WBV) training has been conducted as an effective exercise program for older adults (Machado et al., 2010; Roelants et al., 2004). In this training method, vibration is transmitted to the whole body from the platform to stimulate the muscle spindles, causing a form of muscle contraction named “tonic vibration reflex” (Burke et al., 1978; Hagbarth & Eklund, 1966; Roelants et al., 2004). Vibrations that rapidly change the length of the muscle-tendon complex can promote blood circulation, increase the sensitivity of sensory receptors by contributing to muscle activation, and improve neuromuscular performance (Cardinale & Bosco, 2003; Issurin & Tenenbaum, 1999). WBV training does not require a large amount of physical space because its range of motion is not wide. WBV is also convenient and safe because it can produce an exercise effect without adding additional weight to the body. Furthermore, WBV training results in less fatigue than other exercises (Chanou et al., 2012). Because difficult movements are not required in WBV, it is easy for beginners or older adults to conduct WBV training independently, without the help of others. As such, WBV training is an attractive exercise program that can produce practical and lasting effects in older adults with low motivation to participate in exercise (Delecluse et al., 2003).
WBV training has recently gained popularity among the older adults for rehabilitation and physical performance enhancement. WBV training has a positive impact on muscle mass and strength in older adults (Bogaerts et al., 2007; Machado et al., 2010) and is as effective as resistance exercise in improving muscle function (Bogaerts et al., 2007; Delecluse et al., 2003; Madou & Cronin, 2008; Verschueren et al., 2004). However, some studies have reported contradictory evidence regarding the effectiveness of WBV training (Bautmans et al., 2005; Cristi et al., 2014; Gómez-Cabello et al., 2013; Rees et al., 2008). Rees et al. (2008) demonstrated that WBV training improved the muscle strength and power of the ankle plantar flexors, but not the strength of the knee flexors and extensors. Furthermore, Cristi et al. (2014) showed that 9 weeks of WBV for older adults did not have a significant impact on flexibility. Although several meta-analyses have been conducted to provide evidence of the effectiveness of WBV training (Osawa et al., 2013; Pessoa et al., 2017; Rogan et al., 2015), these studies have some limitations. First, none of the physical fitness components were analyzed. In this regard, most studies have focused on muscle function but not on muscle endurance (Osawa et al., 2013; Pessoa et al., 2017; Rogan et al., 2015). In addition, studies that analyzed variables other than muscle function were limited to the literature review level (Merriman & Jackson, 2009). Thus, meta-analyses of flexibility variables are lacking. Second, despite the impact of moderating variables such as exercise type and duration on WBV training, previous reviews did not consider these factors in the recommendation of guidelines for WBV training programs in older adults. Third, the classification of the measurement variables was ambiguous. Some studies have defined measurement variables that can be interpreted as muscle endurance or flexibility as “functional movements” (Bautmans et al., 2005; Cheung et al., 2007; Cristi et al., 2014; Merriman & Jackson, 2009). For example, the five-times sit-to-stand test or 30-second chair stand test can be considered as measurements of muscle endurance, and the sit-and-reach or back scratch test can be considered as measurements of flexibility. Adequate classification of physical fitness levels is important for suggesting and/or establishing the effectiveness of WBV training in both older adults and clinicians. Therefore, we aimed to update the evidence on the effects of WBV training in older adults by reviewing the literature published to date.
In light of the limitations of previous reviews, the purpose of this meta-analysis was as follows: First, to investigate the effectiveness of WBV training on five aspects of muscle function (strength, power, muscle endurance, functional lower-extremity flexibility [FLEF], and functional lower-extremity strength [FLES]) and second, to determine whether WBV training could improve muscle function over various training periods (10-day, short-term, intermediate-term, and long-term) in older adults.
Materials and Methods
Literature Search
A comprehensive literature search was performed to identify peer-reviewed journal articles on the effectiveness of WBV training for improving strength, power, muscle endurance, functional flexibility, and functional strength in older adults. The studies used in this meta-analysis were identified from electronic databases, including PubMed, SPORTDiscus, Web of Science, and CINAHL, from their inception until April 2022. Two independent authors (HJK and HGJ) systematically searched and screened the relevant studies. The literature search included the keywords (“whole-body vibration” OR WBV) AND (strength OR “repetition maximum” OR torque OR endurance OR power OR jump OR flexibility OR ROM OR “range of motion”) in each database. The reference lists of pooled studies were also searched for other relevant articles. The search was limited to studies involving humans, written in English and reported in peer-reviewed journals. Literature selection was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart (Moher et al., 2009) shown in Figure 1.
Eligibility Criteria
The eligibility of the studies identified in the systematic search was assessed using the inclusion and exclusion criteria described in the following paragraphs.
The inclusion criteria used to select studies were as follows:
The primary purpose of this study was to investigate the effect of WBV training on lower-extremity strength, power, muscle endurance, FLEF, and FLES.
Participants must be older adults (age ≥ 65 years). Since a previous review examined the evidence for defining older adults (Orimo et al., 2006), this review defined an “older adult” as a person aged 65 years or more.
The study must have reported descriptive statistics such as means, standard deviations, and sample size.
The study conducted WBV training for at least 10 days.
The exclusion criteria were as follows:
The study only reported the acute effects of WBV training.
WBV training was conducted in participants with musculoskeletal injuries, diseases, or on oral medications. However, sarcopenia was not classified as an injury in our study because sarcopenia is a common characteristic of aging (a study of older adults with sarcopenia was included in this review).
The study conducted WBV training with additional treatments, such as intake of creatine and vitamin D.
The study had vibration equipment only on the local segments or joints.
Study Selection
We investigated five aspects of lower-extremity muscle function: (1) strength, (2) power, (3) endurance, (4) FLEF, and (5) FLES. Studies reporting isokinetic, isometric, and isotonic contractions of the lower-extremity, such as hip extension and flexion, knee extension and flexion, and ankle dorsiflexion and plantar flexion, were included to examine muscle strength. Muscle endurance was examined through maximal repetition of tasks, including chair stand, and knee flexion and extension. Muscle power was evaluated by an assessment task that required maximal force in a short period of time, such as hip flexion and extension, knee extension and flexion, ankle dorsiflexion and plantar flexion, and countermovement jump. The chair stand-and-reach and functional reach tests were used to investigate FLEF in older adults. The chair stand-and-reach test and functional reach test, as functional fitness tests for older adults, are valuable tools for measuring FLEF in older adults, and their reliability was valid (Duncan et al., 1990; Rikli & Jones, 1999). The sit-to-stand test was conducted five times to measure the FLES scores of older adults (Melo et al., 2019). Therefore, studies reporting five sit-to-stand test results were also included in this review to investigate lower-extremity function associated with the strength of older adults.
Assessment of Methodologic Quality
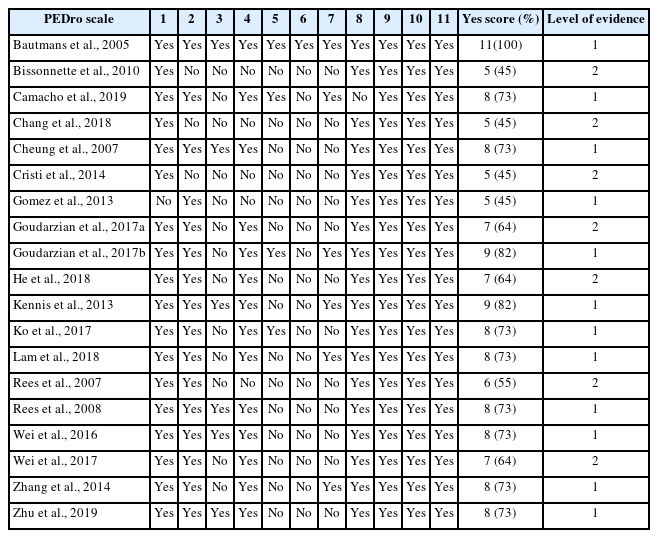
The Physiotherapy Evidence Database (PEDro) (Sherrington et al., 2010) and strength of recommendation taxonomy (SORT) (Ebell et al., 2004) were used as assessment tools, with high reliability and validity for the quality of the included studies. The checklist included 11 questions and indicated the total score as a percentage. Two authors independently assessed the full texts of the selected studies for quality analysis. Discrepancies in screening and scoring were addressed collaboratively until a consensus was reached. Disagreements were resolved by two other authors. The selected studies were classified into levels 1 to 3 according to their quality. In the current review, evidence level 1 was considered a PEDro scores 70%, evidence level 2 as > 40% and < 70%, and evidence level 3 as PEDro scores ≤ 40% (Sherrington et al., 2010). The SORT recommendations were used to grade the quality of evidence. The SORT reports grade A as “consistent and good quality patient-oriented evidence,” grade B as “inconsistent or limited quality patient-oriented evidence,” and grade C as “consensus, usual practice, opinion, disease-oriented evidence, and case series for studies of diagnosis, treatment, prevention, or screening” (Ebell et al., 2004).
Assessment of Publication Bias
After reviewing the data from this meta-analysis using a forest plot, the asymmetry of the effect size was visually evaluated using a funnel plot. In addition, the relationship between effect size and standard error was verified using Egger’s regression to determine whether the funnel plot was asymmetric. For asymmetric data, we calculated the average effect size, obtained by adjusting the asymmetry using the trim-and-fill method, and compared it with the original average effect size.
Effect Size Analysis
Since the use of standardized mean difference (SMD, Cohen’s d) to calculate the effect size can result in overestimation with a small sample size, Hedges’ adjusted g was used in this study with the following criteria: g of .2 or less was considered as a small effect, g between .2 and .8 was considered as a medium effect, and g greater than .8 was considered as a large effect. To calculate the SMD and 95% confidence intervals (CIs), means, standard deviations pre- and post-intervention, and numbers were extracted from each study. The entire review process was performed by two authors, including the assessment of the aims and quality of the studies, characteristics and inclusion criteria of participants, intervention procedures, and outcome variables. The heterogeneity level was determined using the I2 value: high (I2 ≥ 75%), medium (25% < I2 < 75%), and low (I2 < 25%). A random-effects model was used in this meta-analysis to generate summary results of independently conducted studies. RevMan (Version 5.3.5) was used for the meta-analysis, and R (version 4.0.2) with the “metafor” package was used for publication bias assessment. The CIs for the effect size were 95%, and the significance level was set at .05.
Results
Study Selection
The flowchart presented in Figure 1 follows PRISMA guidelines. The initial search yielded 2,761 relevant studies. After screening, 16 studies were included in this meta-analysis. Furthermore, an examination of the references of the pooled studies identified three additional papers. These studies were used to determine whether WBV training could improve physical fitness. A total of 474 participants (mean age 74.27 years) were included in the final analyses of all 19 studies. In this meta-analysis, the WBV training periods varied among the different studies. Therefore, we classified the training periods into 10-day, short-term (4 to 8 weeks), intermediate-term (9 to 18 weeks), and long-term (over 1 year) categories and conducted a subanalysis according to each category. In this review, 10-day and short-term training periods were distinguished. Despite the evidence that 10 days of WBV training was effective on muscle function by increasing neuromuscular adaptation, both periods were labeled “short-term training periods” in several previous studies. Therefore, we classified the two periods into different subgroups and verified the effect of WBV according to the training period. A methodological summary of the included studies is shown in Table 1.
Level of Evidence and Strength of Recommendation
Seven studies (Bissonnette et al., 2010; Chang et al., 2018; Cristi et al., 2014; Goudarzian, Rahimi, et al., 2017; He et al., 2018; Rees et al., 2007; Wei et al., 2017) were classified as Level 2, with an average PEDro score of 7.0. Twelve studies (Bautmans et al., 2005; Camacho-Cardenosa et al., 2019; Cheung et al., 2007; Gómez-Cabello et al., 2013; Goudarzian, Rahimi, et al., 2017; Kennis et al., 2013; Ko et al., 2017; Lam et al., 2018; Rees et al., 2008; Wei et al., 2016; Zhang et al., 2014; Zhu et al., 2019) were classified as level 1 with an average PEDro score of 7.7 (Table 2) (Sherrington et al., 2010). Regarding the grading of variables, grade A was assigned when consistent results were obtained in at least two high-quality randomized controlled trial (RCT) studies. Therefore, the variables of strength, muscle power, and muscle endurance were classified as grade A, whereas the variables of FLEF and FLES were classified as grade B.
Assessment of Publication Bias
Egger’s regression analysis was conducted to determine the symmetry of funnel plots (Figure 2). The analysis confirmed that the variables of muscle power (p = .49) and FLEF (p = .11) showed symmetry, with no publication errors. In contrast, asymmetry of muscle strength (p < .01), muscle endurance (p < .01), and FLES (p < .01) variables were observed, and publication errors were confirmed. Therefore, we applied the trim-and-fill method to these three variables. The effects of publication errors on the research results were confirmed. A comparison of the existing SMD with the adjusted SMD showed that the difference was less than 10%. Therefore, the effects of publication errors in power, muscle endurance, and FLES variables on the results of the study were small.
Data Synthesis
Muscle Strength
Figure 3 shows the overall effect size measures for the impact of WBV training on strength in older adults using a forest plot (Q (48) = 92.24, p < .001, I2 = 48%). Under the random-effects model, the overall difference in WBV training on muscle strength was statistically significant (SMD = .54, z = 7.92, p < .001, 95% CI = .40, .67, k = 49), indicating that WBV training could improve muscle strength in older adults compared with that in the pre-WBV training condition (with a medium effect size).
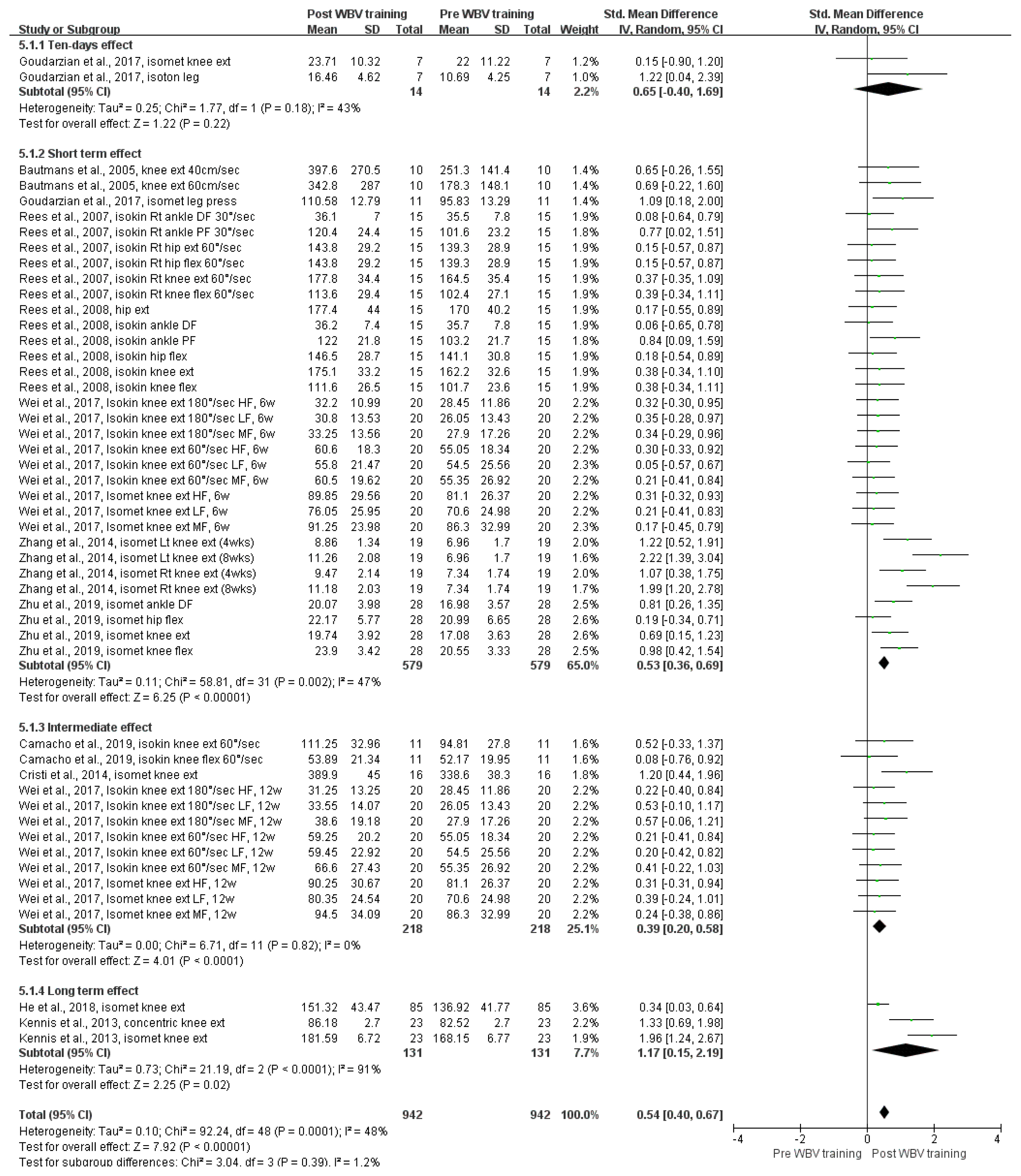
Forest plots of the SMD for the meta-analysis of the impact of WBV training on muscle strength. Abbreviations: CI, confidence interval; DF, dorsiflexion; ext, extension; flex, flexion; HF, high frequency; isokin, isokinetic; isomet, isometric; isoton, isotonic; LF, low frequency; Lt, left; MF, medium frequency; PF, plantarflexion; Rt, right; SD, standard deviation; SMD, standardized mean difference; WBV, whole-body vibration
A subgroup analysis was conducted to evaluate the impact of different WBV training periods on strength: 10-day (k = 2), short-term (k = 32), intermediate-term (k = 12), and long-term (k = 3). The overall difference in WBV training times on muscle strength was statistically significant for the following training periods: short-term (SMD = .53, 95% CIs = .36, .69, p < .001), intermediate-term (SMD = .39, 95% CIs = .20, .58, p < .001), and long-term (SMD = 1.17, 95% CIs = .15, 2.19, p = .02). However, there was no statistically significant difference in the training period of 10 days (SMD = .65, 95% CIs = −.40, 1.69, p = .22). Therefore, in comparison with the pre-WBV training condition in older adults, short-term and intermediate-term WBV training had a medium effect size on strength improvement, and long-term WBV training had a large effect size on strength improvement.
Power
Figure 4 shows the overall effect size measures for the impact of WBV training on power in older adults using a forest plot (Q (15) = 35.98, p = .002, I2 = 58%). Under the random-effects model, the overall difference in WBV training on power was statistically significant (SMD = .58, z = 3.73, p < .001, 95% CIs = .28, .89, k = 16), indicating that WBV training could improve power in older adults compared to that in the pre-WBV training condition (with a medium effect size).
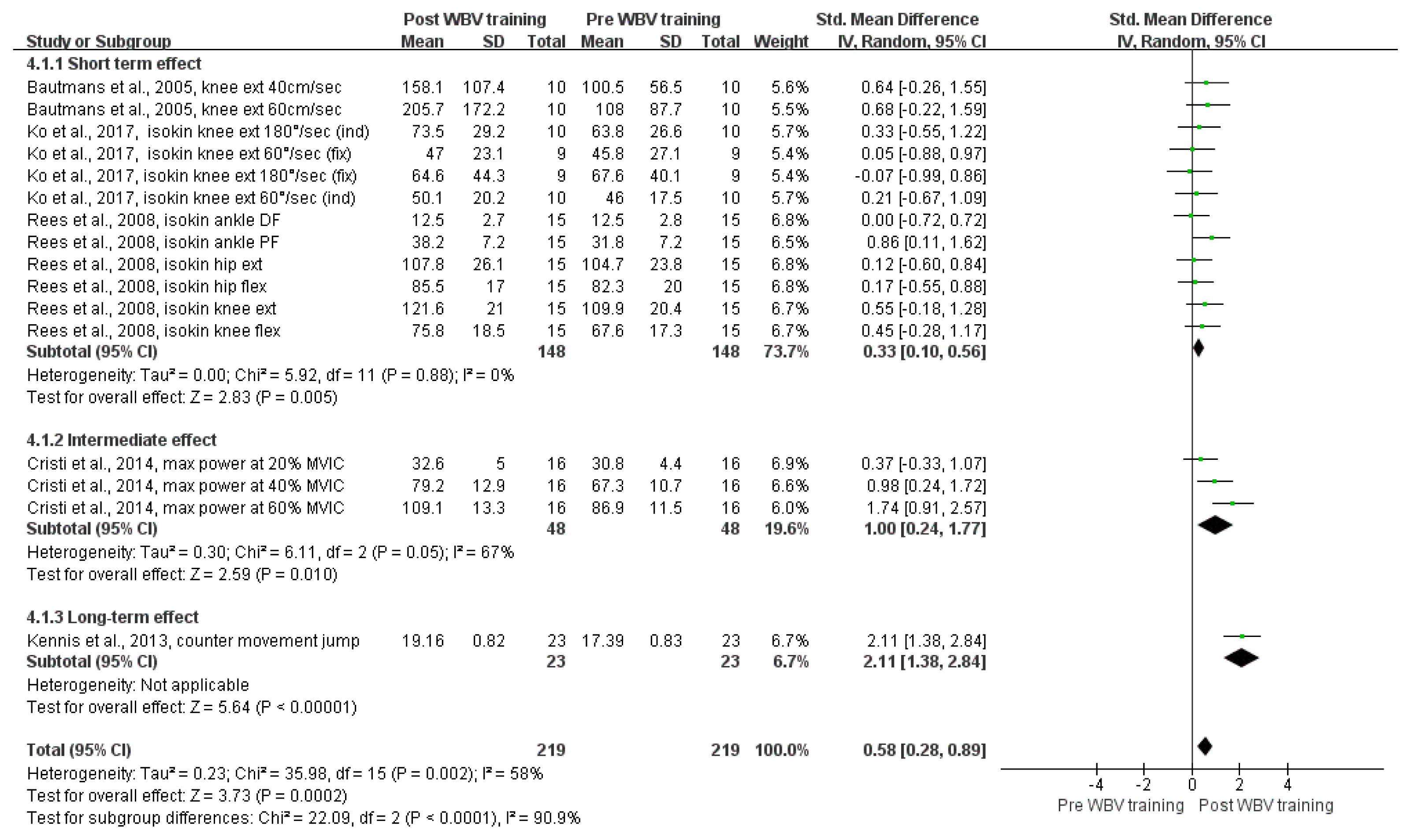
Forest plots of the SMD for the meta-analysis of the impact of WBV training on power. Abbreviations: CI, confidence interval; DF, dorsiflexion; ext, extension; flex, flexion; MVIC, maximal voluntary isometric contraction; PF, plantar flexion; SD, standard deviation; SMD, standardized mean difference; WBV, whole-body vibration
A subgroup analysis was conducted to evaluate the impact of different WBV training periods on power: short-term (k = 12), intermediate-term (k = 3), and long-term (k = 1). The overall difference in WBV training times on power was statistically significant for the following training periods: short-term (SMD = .33, 95% CIs = .10, .56, p = .005), intermediate-term (SMD = 1.0, 95% CIs = .24, 1.77, p = .01), and long-term (SMD = 2.11, 95% CIs = 1.38, 2.84, p < .001). Therefore, in comparison to the pre-WBV training condition in older adults, short-term WBV training had a medium effect size on power improvement, while intermediate- and long-term WBV training had large effect sizes on power improvement.
Muscle Endurance
Figure 5 shows the overall effect size measures of the impact of WBV training on muscle endurance in older adults using a forest plot (Q (8) = 27.71, p < .001, I2 = 71%). Under the random-effects model, the overall difference in WBV training on muscle endurance was statistically significant (SMD = .82, z = 3.50, p < .001, 95% CIs = .36, 1.29, k = 9), indicating that WBV training could improve muscle endurance in older adults compared to that in the pre-WBV training condition (with a large effect size).

Forest plots of SMD for the meta-analysis of the impact of WBV training on muscle endurance (maximum repetition number). Abbreviations: CI, confidence interval; Ext, extension; flex, flexion; isokin, isokinetic; SD, standard deviation; SMD, standardized mean difference; WBV, whole-body vibration
A subgroup analysis was conducted to evaluate the impact of different WBV training periods on muscle endurance: short-term (k = 5) and intermediate-term (k = 4). The overall effect of WBV training times on muscle endurance was statistically significant for the short-term training period. (SMD = .80, 95% CIs = .36, 1.24, p < .001). However, no statistically significant difference was found in the intermediate-term training period (SMD = .89, 95% CIs = −.14, 1.92, p = .09). Therefore, in comparison to the pre-WBV training condition in older adults, only short-term WBV training had a large effect on muscle endurance improvement.
Functional Lower-extremity Flexibility (FLEF)
Figure 6 shows the overall effect size measures for the impact of WBV training on FLEF in older adults using a forest plot (Q (5) = 3.08, p = .69, I2 = 0%). Under the random-effects model, the overall difference in WBV training on FLEF was statistically significant (SMD = .31, z = 2.47, p = .01, 95% CIs = .06, .55, k = 6), indicating that WBV training could improve FLEF in older adults compared to that in the pre-WBV training condition (with a medium effect size).

Forest plots of SMD for the meta-analysis of the impact of WBV training on functional lower-extremity flexibility. Abbreviations: CI, confidence interval; CSAR, chair stand-and-reach; SD, standard deviation; SMD, standardized mean difference; WBV, whole-body vibration
Subgroup analysis was conducted to evaluate the impact of different WBV training periods on FLEF: short-term (k = 3) and intermediate-term (k = 3). The overall difference in WBV training time on FLEF was statistically significant for the short-term training period (SMD = .50, 95% CIs = .09, .91, p = .02). However, no statistically significant difference was found in the intermediate-term training period (SMD = .20, 95% CIs = −.10, .50, p = .20). Therefore, compared to the pre-WBV training condition in older adults, only short-term WBV training had a medium effect size on FLEF improvement.
Functional Lower-Extremity Strength (FLES)
Figure 7 shows the overall effect size measures for the impact of WBV training on FLES in older adults using a forest plot (Q (5) = 9.98, p = .08, I2 = 50%). Under the random-effects model, the overall difference in WBV training on FLES was statistically significant (SMD = −.75, z = 3.51, p < .001, 95% CIs = −1.18, −.33, k = 6), indicating that WBV training could improve FLES in older adults as compared to that in the pre-WBV training condition (with a medium effect size).

Forest plots of SMD for the meta-analysis of the impact of WBV training on functional lower-extremity strength. Abbreviations: CI, confidence interval; Reps, repetitions; SD, standard deviation; SMD, standardized mean difference; WBV, whole-body vibration
For FLES, short-term (k = 3) and intermediate-term (k = 3) WBV training periods were included in the subgroup analysis. The overall difference in the impact of WBV training times on FLES was statistically significant for the short-term training period (SMD = −1.07, 95% CIs = −1.54, −.59, p < .001). However, no statistically significant difference was found in the intermediate-term training period (SMD = −.48, 95% CIs = −1.0, .03, p = .07). Therefore, in comparison to the pre-WBV training condition in older adults, only short-term WBV training had a large effect on FLES improvement.
Discussion
The purpose of this systematic review and meta-analysis was to collect, appraise, and synthesize the results of the current literature or evidence in which researchers have demonstrated the impact of WBV training on muscle function, including strength, power, muscle endurance, FLEF, and FLES in older adults. We further provided optimal parameters and WBV training protocols based on the results to improve muscle function. This meta-analysis confirmed the following primary findings: 1) WBV training is an effective intervention for improving muscle function in older adults; 2) the impact of WBV exercise was the greatest on muscle endurance, followed by FLES, power, strength, and FLEF, and 3) as the training period of WBV increased, its impact on strength and power increased in older adults. High-quality (grades A and B) evidence supported these results, as indicated by the consistent presence of Level 1 and 2 evidence from individual studies.
Muscle Endurance
The present study found grade A evidence (SORT) of muscle endurance with WBV training, which demonstrated the greatest improvement (SMD = .82) among all five muscle functions. Regarding muscle endurance, most studies reported this variable during chair stand and isokinetic knee flexion and extension tests. Based on the subanalysis of the impact of short-term and intermediate-term WBV training in this study, the 4-week WBV training demonstrated a large effect size (SMD = .80) on endurance improvement. Therefore, a minimum of 4-week WBV training is recommended to improve muscle endurance.
Furthermore, based on the exercise protocol of the study that showed statistical significance, static and dynamic exercises on vibration plates, such as calf raise, squat, and lunge, are recommended to increase the muscle endurance of the lower extremities in older adults. However, since repeated vibration may temporarily inhibit the proprioceptive function of the lower-extremity, basic strength and balance ability should be confirmed before initiating dynamic WBV exercises to prevent falls.
Two muscle endurance variables showed statistically significant results during the intermediate-term WBV training period (Cristi et al., 2014; Gómez-Cabello et al., 2013). The common parameter of WBV training in these two studies (Cristi et al., 2014; Gómez-Cabello et al., 2013) was the high frequency of the vibration plate (30–45 Hz in Cristi et al., 40 Hz in Gomez et al.). In contrast, a low vibration frequency of 12.6 Hz (Camacho-Cardenosa et al., 2019) did not show a statistically significant improvement in muscle endurance after WBV training. This observation could be explained by the fact that the high frequency and amplitude of the vibration activate energy by increasing maximal oxygen consumption (Rittweger et al., 2002, 2003). Consequently, neuromuscular control and electromyographic activation also increase (Ritzmann et al., 2014). Increased electromyography activity allows extensive use of muscle fibers, resulting in improved muscle endurance (Aagaard, 2003; Ritzmann et al., 2013). However, since the percentage of fast muscle fibers decreases and that of slow muscle fibers increases with age, the impact of vibration is more powerful for older adults. Because muscle endurance is closely related to the oxidative capacity of slow muscle fibers (Rittweger et al., 2003), a larger effect may be observed in older adults with a relatively high ratio of slow muscle fibers. Consequently, because the effect size of muscle endurance was the largest among all muscle functions in this study, endurance was the muscle function that WBV training could improve the most.
Power
We found grade A evidence (SORT) that WBV training could improve power in older adults (SMD = .58, indicating increased power). Pooled studies in this review investigated the impact of WBV training on lower-extremity power by analyzing hip, knee, and ankle joint power as well as functional tasks, such as the countermovement jump. The most important finding related to this variable was that increasing the WBV training time resulted in a large effect size on power improvement. Therefore, it is appropriate to increase the duration of WBV training to increase lower-extremity muscle power in older adults. In this meta-analysis, the muscle power variable showed a medium effect size, indicating that WBV training is recommended as an exercise to improve lower-extremity muscle power in older adults.
Several studies (Bautmans et al., 2005; Ko et al., 2017; Rees et al., 2008) have analyzed power in the subanalysis of short-term WBV training. However, the impact of short-term WBV training on muscle power was negligible (SMD = .33), compared to that of intermediate- and long-term WBV training. Only Rees et al. (2008) reported statistically significant and large effect size (ankle plantar flexor power, SMD = .86) on power improvement after short-term WBV training. In addition, no significant difference was found in other analyses of the muscle power of the lower-extremity, including ankle dorsiflexion, hip extension, hip flexion, knee extension, and knee flexion. Additionally, studies investigating the impact of short-term WBV training on muscle power have reported different joint-specific results. Therefore, it is difficult to present the common training characteristics.
Despite the limited number of studies on muscle power, in a subanalysis of intermediate-term WBV training, Cristi et al. (2014) demonstrated that the improvement in power by WBV training increased with the level of the measurement task. These findings of the sequential difference in the effect size suggest that different muscle contractility is required at each level of the measurement task and that WBV training is recommended for older adults who want to participate in high-level sports. However, due to the limited number of studies and the lack of diversity in measured variables, the improvement in lower-extremity power through intermediate-term WBV training has not been well demonstrated.
Our subanalysis of the impact of long-term WBV training on power included only one study (Kennis et al., 2013). Kennis et al. (2013) demonstrated the impact of long-term WBV training on lower-extremity power through a countermovement jump task. The effect size of this variable was the largest among the variables analyzed in this review (SMD = 2.11). Since comparisons with other studies or other measurement variables with no statistical significance were not possible, the parameters with a significant influence on power in this study remain unknown. Nevertheless, a long WBV training duration of one year showed statistically significant and clinically meaningful improvements in functional movement in older adults. Therefore, long-term WBV training can be a useful intervention for older adults participating in sports by improving their functional movements and activities of daily living.
Strength
This study found grade A evidence (SORT) that WBV training could improve strength in older adults (SMD = .52, indicating increased strength). Muscle strength was included in most of the pooled studies in this review. In these studies, the knee, hip, and ankle joints were examined. Various examination protocols, such as isometric, isokinetic, and isotonic, have been used to determine the type of contraction. Notably, the effect size of WBV training on strength improvement increased with WBV training duration. Except for the 10-day training period, both short-term and intermediate-term WBV training significantly improved muscle strength. Furthermore, the greatest improvement in strength was observed with long-term WBV training.
In this review, the impact of 10-day WBV training on muscle strength was examined. Only one study was included in this short-term subanalysis, which did not show a statistically significant improvement in strength with WBV training (Goudarzian, Ghavi, et al., 2017). An acute impact of WBV on muscle function has also been reported (Cormie et al., 2006). However, a training protocol with sufficient duration is required to verify the effectiveness of WBV because it is difficult to change the nature of the muscle with short-term exposure to vibrations. Further research is warranted to determine whether the 10-day or extremely short WBV intervention is clinically meaningful in improving muscle strength in older adults.
In a subanalysis of the effect of short-term WBV training on muscle strength, five studies (Goudarzian, Rahimi, et al., 2017; Rees et al., 2007; Rees et al., 2008; Zhang et al., 2014; Zhu et al., 2019) showed statistically significant increases in muscle strength after WBV training. Although no common characteristics of the WBV training protocol and parameters were found among these studies, a high training volume (at least three sets, three to five training sessions per week) should be noted. In addition, a difference in vibration frequencies was observed in some WBV training studies. In this regard, the vibration frequency in the study by Ree et al. (2007) was the lowest (26 Hz, on average) compared to those of Bautmans et al. (2005) (30–40 Hz) and Wei et al. (2017) (20–60 Hz). However, it remains unclear whether differences in the vibration frequency of WBV training contribute to these findings.
Since two studies (Zhang et al., 2014; Zhu et al., 2019) included the oldest WBV training participants (over 85 years of age), only static exercise was examined. However, a large effect size was observed in the WBV training protocol five times per week. Therefore, an age-specific training protocol should be considered when training individuals with advanced age and high-risk injuries. In addition, the study by Zhang et al. (2014) had an exceptionally large effect size (SMD = 2.22 and 1.99) compared with that in other studies. Another interesting characteristic of this study was that all the participants were older adult men. Although some sex-dependent differences, such as movement pattern and surface electromyography, have been demonstrated (Sañudo et al., 2012), further evidence is required to determine the influence of sex on the effectiveness of WBV training on muscle strength.
In a subanalysis of intermediate-term WBV training, only one study (Cristi et al., 2014) showed a significant improvement in muscle strength, with a large effect size (SMD =1.20). Although the training period of this study was shorter (9 weeks) than that of the other two studies (12–18 weeks) (Camacho-Cardenosa et al., 2019; Wei et al., 2017) in the intermediate-term analysis, it effectively improved muscle strength in older adults. The selection of exercise items in the WBV training protocol may have contributed to the differences in the strength improvement outcomes among the studies. In this regard, Cristi et al. (2014) included dynamic tasks, such as deep squats, wide stance squats, calf raises, and static squat exercises in the WBV training protocol. Interestingly, one study (Wei et al., 2017) investigated the impact of WBV training at various vibration frequencies. However, there was no difference in muscle strength improvement among the three vibration frequencies examined in this study (20, 40, and 60 Hz).
Long-term WBV training was significantly more effective in improving muscle strength in older adults than a relatively shorter training period, including the 10-day, short-, and intermediate-term periods. All studies (He et al., 2018; Kennis et al., 2013) have demonstrated increased muscle strength in older adults after long-term WBV training. By analyzing the characteristics of these studies, some common parameters were identified as follows: static exercises (squat, deep squat, wide stance squat, lunge, toes stand, toes stand deep, moving heels) and dynamic exercises (slowly going up and down in 2 s); training period of 1 year; training protocol of three times per week, with 4–15 sets of exercises; vibration duration of 30–60 s, rest duration of 30–60 s, vibration amplitude of 2.5 or 5 mm, vibration frequency of 35–40 Hz, and vertical-synchronous vibration. These parameters can serve as a guideline for improving the strength of older adults through long-term WBV training for one year.
Functional Lower-Extremity Flexibility (FLEF)
This study found grade B evidence (SORT) that WBV training could improve FLEF in older adults (SMD = .31, indicating increased FLEF). Most studies have examined FLEF through the chair sit-and-reach task, with the exception of one study that used functional reach (Cheung et al., 2007). The present review demonstrated that the effect size of the FLEF variable was the lowest compared to other muscle functions, including strength, power, muscle endurance, and FLES, in older adults after WBV training. There was little difference in the effect size of FLEF between the short-term (SMD = .50) and intermediate-term (SMD = .20) WBV training. This result has important implications for both clinicians and older adults from two perspectives. First, excessively long WBV training periods were not required to improve FLEF in older adults. Second, there may be limitations to improving FLEF with WBV training alone. Therefore, in addition to WBV training, other exercise programs designed specifically for older adults should be considered. Furthermore, the short-term subanalysis included WBV training periods of 4, 6, and 8 weeks. However, only 8 weeks of WBV training demonstrated a statistically significant improvement in FLEF in older adults. Therefore, a minimum of 8 weeks of WBV training is recommended to increase FLEF in older adults.
Functional Lower-Extremity Strength (FLES)
Our study found grade B evidence (SORT) that WBV training could improve FLES in older adults (SMD = −.75, indicates increased FLES). All studies confirmed the impact of WBV training on FLES using the sit-to-stand task. In the short-term subanalysis of the FLES, two studies showed statistical significance. The most important factor influencing the effectiveness of short-term WBV training in FLES may be the exercise type. Rees et al. (2007) and Goudarzian, Rahimi et al. (2017) analyzed dynamic exercises such as lunges, calf raises, and up and down squats. Conversely, Wei et al. (2016) analyzed static squat exercises on a vibrating plate and did not observe an improvement in FLES with WBV training. Therefore, similar to other muscle function variables, the training protocol must consist of dynamic exercise items to improve the FLES in older adults.
In the subanalysis of FLES, a smaller effect size was observed in intermediate-term training than in short-term WBV training. As observed in the aforementioned results for the FLEF variable, the effectiveness of WBV training decreased with increased training duration. The precise underlying mechanism of this phenomenon, although unclear to the authors, might be partly due to the limitations of WBV training in improving all muscle functions in older adults. Since statistical significance was observed in only one study (Wei et al., 2016), it is difficult to recommend meaningful guidelines.
Since proprioception decreases with age in older adults, the movement pattern and neuromuscular control of the lower extremities decrease, implying poor balance and increased risk of injury, such as falls (Ferlinc et al., 2019). However, traditional training, such as strength and endurance exercises, cannot improve neuromuscular control in older adults, even after a long training period of 6 months (Wilhelm et al., 2014). Therefore, the most important clinical application of our findings in the FLES variable is that short-term WBV training can improve functional movement in older adults.
Clinical Implications and Future Research
The most important finding of the present meta-analysis was that WBV training can effectively improve various aspects of muscle function in older adults. In addition to these guidelines, information regarding WBV training for older adults is available. Future studies should examine the post-exercise retention impact of WBV training on muscle function and whether different training durations could influence the long-term impact of WBV training on muscle function. As such, an abundance of clinically useful information about WBV exercises will be available for the recommendation of a proper exercise protocol for older adults.
Limitations
Although our meta-analysis with a systematic review followed the PRISMA guidelines, there are some limitations. First, a few studies used 10-day and long-term WBV training to confirm the results of the subgroup analysis. Therefore, additional evidence should be collected to determine the effectiveness of extremely short or long WBV training sessions on muscle function in older adults. Second, although some RCTs were included in this review, the average methodological quality of all the studies was not high. Additionally, many studies failed to blind all participants (15 of 19 studies) and therapists who administered the training intervention (18 of 19 studies). Future studies should seek to improve upon these limitations to enhance the level of evidence and the strength of recommendations. Third, although two authors conducted a systematic literature search, there were limitations in the search and screening process. The number of search keywords was limited to suggest stronger evidence, and detailed reasons were not provided for excluding studies based on our exclusion criteria.
Conclusions
The conclusions of our systematic review and meta-analysis are as follows: 1) WBV training improves muscle function, including strength, power, muscle endurance, and FLEF in older adults; 2) WBV training has the greatest impact on muscle endurance; 3) there is a difference in the effect size according to WBV training duration for each variable of muscle function, and the recommendation of the WBV training period to improve strength, muscle endurance, and power is at least 8 weeks, and 4) the longer the WBV training period, the more effective it is to improve muscle endurance, power, and strength, but not flexibility and FLES, in older adults. Furthermore, we provide detailed recommendations, such as the training period, exercise type, and parameters of WBV training for each variable of the muscle function. However, further systematic explorations are required to investigate whether a long-term impact of WBV training exists and whether it is influenced by the training duration.
Acknowledgments
We thank the Institute of Convergence Science (ICONS) of Yonsei University and the International Olympic Committee (IOC) Research Centre Korea for Prevention of Injury and Protection Athlete Health.
Notes
Author Contributions
Conceptualization: H.J.K & H.G.J & S.Y.L
Data curation: H.J.K & S.E.P & H.G.J
Formal analysis: H.J.K & S.E.P & H.G.J
Investigation: H.J.K & S.E.P & H.G.J
Project administration: H.G.J & S.Y.L
Writing—original draft preparation: H.J.K & S.E.P
Writing—review and editing: H.G.J & S.Y.L
Conflict of Interest
This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors. This study did not require ethical approval because it was a secondary data analysis. The authors report there are no competing interests to declare.